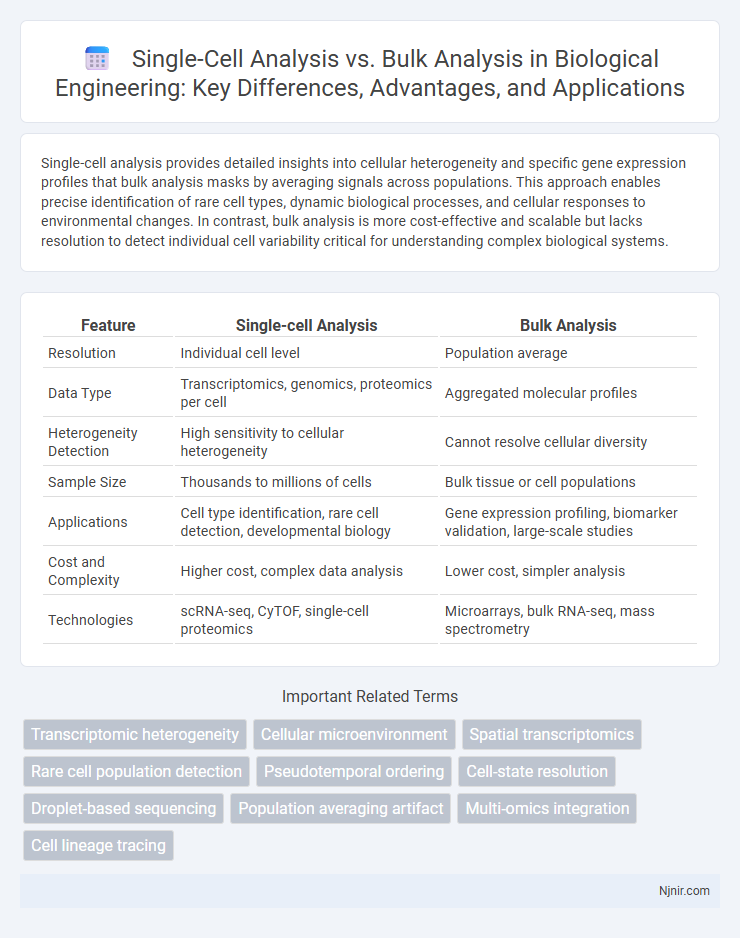
Single-cell analysis provides detailed insights into cellular heterogeneity and specific gene expression profiles that bulk analysis masks by averaging signals across populations. This approach enables precise identification of rare cell types, dynamic biological processes, and cellular responses to environmental changes. In contrast, bulk analysis is more cost-effective and scalable but lacks resolution to detect individual cell variability critical for understanding complex biological systems.
Table of Comparison
| Feature | Single-cell Analysis | Bulk Analysis |
|---|---|---|
| Resolution | Individual cell level | Population average |
| Data Type | Transcriptomics, genomics, proteomics per cell | Aggregated molecular profiles |
| Heterogeneity Detection | High sensitivity to cellular heterogeneity | Cannot resolve cellular diversity |
| Sample Size | Thousands to millions of cells | Bulk tissue or cell populations |
| Applications | Cell type identification, rare cell detection, developmental biology | Gene expression profiling, biomarker validation, large-scale studies |
| Cost and Complexity | Higher cost, complex data analysis | Lower cost, simpler analysis |
| Technologies | scRNA-seq, CyTOF, single-cell proteomics | Microarrays, bulk RNA-seq, mass spectrometry |
Introduction to Single-Cell and Bulk Analysis
Single-cell analysis examines the genetic, transcriptomic, and proteomic profiles of individual cells, enabling precise insights into cellular heterogeneity and rare cell populations. Bulk analysis aggregates data from thousands to millions of cells, providing averaged molecular information that masks individual cell variability but is useful for identifying dominant biological signals. Advances in single-cell sequencing technologies have revolutionized research by uncovering cellular diversity unnoticed in traditional bulk methods.
Fundamental Principles of Single-Cell Analysis
Single-cell analysis enables the examination of gene expression, protein levels, and cellular functions at the individual cell level, contrasting with bulk analysis that averages signals across heterogeneous cell populations. This method relies on techniques such as flow cytometry, single-cell RNA sequencing (scRNA-seq), and microfluidics to isolate and analyze cells independently. By capturing cellular heterogeneity, single-cell analysis provides insights into complex biological processes, developmental pathways, and disease mechanisms that bulk analysis often obscures.
Overview of Bulk Analysis Techniques
Bulk analysis techniques measure average molecular signals from large populations of cells, providing insights into gene expression, protein levels, and metabolite concentrations across tissues or cell mixtures. Common bulk methods include RNA-sequencing (RNA-seq), microarrays, and mass spectrometry-based proteomics, which generate comprehensive datasets but mask cellular heterogeneity. These techniques excel in detecting overall biological trends and regulatory mechanisms but lack the resolution to identify cell-specific variations present in complex samples.
Key Technological Advancements
Single-cell analysis utilizes advanced microfluidics, next-generation sequencing (NGS), and high-throughput imaging to capture cellular heterogeneity and gene expression profiles at an individual cell level, enabling precise insights into cellular functions and disease mechanisms. Bulk analysis aggregates data from numerous cells, relying on techniques such as RNA sequencing and proteomics that provide average molecular signals but obscure individual cell variability. Innovations like droplet-based sequencing and multiplexed protein assays have propelled single-cell technologies beyond bulk analysis limitations, enhancing resolution in genomics and transcriptomics studies.
Resolution and Sensitivity Comparison
Single-cell analysis offers unparalleled resolution by profiling gene expression, protein levels, and metabolic states at the individual cell level, revealing cellular heterogeneity obscured in bulk analysis. Bulk analysis averages signals from thousands to millions of cells, resulting in lower sensitivity to rare or subtle cellular variations and masking critical cellular subpopulations. Enhanced sensitivity in single-cell techniques enables detection of low-abundance transcripts and rare cell types, providing precise insights into complex biological systems and disease mechanisms.
Applications in Biological Engineering
Single-cell analysis enables the identification of cellular heterogeneity and rare cell populations crucial for precision medicine and developmental biology, while bulk analysis provides average gene expression profiles useful for understanding tissue-level responses. In biological engineering, single-cell methods facilitate the design of targeted therapies and synthetic biology applications by revealing unique cellular states and signaling pathways. Bulk analysis remains valuable for large-scale biomarker discovery and metabolic engineering by offering comprehensive datasets from complex biological samples.
Data Complexity and Interpretation
Single-cell analysis provides high-resolution data by profiling gene expression, protein levels, or genome variations in individual cells, revealing cellular heterogeneity often masked in bulk analysis. Bulk analysis aggregates signals across many cells, simplifying data complexity but limiting interpretation to average molecular profiles, which can obscure rare cell populations or subtle regulatory mechanisms. The increased data complexity in single-cell analysis demands advanced computational tools for dimensionality reduction, clustering, and trajectory inference to accurately interpret cell-specific biological processes.
Advantages and Limitations of Each Approach
Single-cell analysis offers high-resolution insights by examining gene expression and cellular heterogeneity at the individual cell level, enabling the identification of rare cell types and dynamic cellular states. Bulk analysis aggregates data from thousands to millions of cells, providing an averaged molecular profile that is cost-effective and suitable for detecting common genetic signatures but masks cellular diversity. The limitations of single-cell analysis include higher costs, technical complexity, and data sparsity, while bulk analysis struggles with cellular heterogeneity and the loss of single-cell resolution.
Impact on Disease Diagnosis and Therapeutics
Single-cell analysis provides granular insights into cellular heterogeneity and rare cell populations, enabling precise identification of disease biomarkers and personalized therapeutic targets. Bulk analysis averages signals from diverse cell populations, potentially masking critical variations essential for understanding disease mechanisms and treatment responses. Leveraging single-cell technologies enhances diagnostic accuracy and accelerates the development of targeted therapies tailored to individual cellular profiles.
Future Perspectives in Biological Analysis
Single-cell analysis offers unparalleled resolution in understanding cellular heterogeneity, enabling breakthroughs in precision medicine and targeted therapies that bulk analysis cannot achieve. Advances in microfluidics, high-throughput sequencing, and computational biology are driving the evolution of single-cell techniques, promising more comprehensive insights into gene expression, epigenetics, and cellular interactions. The future of biological analysis prioritizes integrating single-cell data with multi-omics and spatial technologies to unravel complex biological systems at unprecedented depth and accuracy.
Transcriptomic heterogeneity
Single-cell analysis reveals transcriptomic heterogeneity by profiling gene expression at individual cell resolution, unlike bulk analysis which averages signals across heterogeneous cell populations.
Cellular microenvironment
Single-cell analysis provides detailed insights into cellular microenvironments by capturing individual cell variations and interactions, unlike bulk analysis which averages signals from heterogeneous cell populations, masking microenvironmental complexity.
Spatial transcriptomics
Spatial transcriptomics in single-cell analysis provides higher resolution gene expression mapping within tissue architecture compared to bulk analysis, enabling precise identification of cellular heterogeneity and spatial gene regulation.
Rare cell population detection
Single-cell analysis enables precise detection and characterization of rare cell populations by profiling individual cells, unlike bulk analysis which averages signals and often obscures these rare subsets.
Pseudotemporal ordering
Single-cell analysis enables precise pseudotemporal ordering of individual cells to reveal dynamic cellular processes, whereas bulk analysis averages signals across populations, obscuring temporal heterogeneity.
Cell-state resolution
Single-cell analysis provides high-resolution insights into individual cell states, revealing cellular heterogeneity that bulk analysis averages and often obscures.
Droplet-based sequencing
Droplet-based sequencing in single-cell analysis enables high-throughput, precise characterization of individual cell transcriptomes, outperforming bulk analysis by revealing cellular heterogeneity and rare cell populations.
Population averaging artifact
Single-cell analysis eliminates population averaging artifacts inherent in bulk analysis by measuring gene expression individually in cells, revealing cellular heterogeneity and rare subpopulations obscured in bulk data.
Multi-omics integration
Single-cell analysis enables precise multi-omics integration by capturing cellular heterogeneity at the transcriptomic, epigenomic, proteomic, and metabolomic levels, whereas bulk analysis averages signals across heterogeneous cell populations, obscuring critical cell-specific interactions.
Cell lineage tracing
Single-cell analysis enables precise cell lineage tracing by capturing individual cellular heterogeneity, whereas bulk analysis averages signals across populations, obscuring lineage-specific variations.
Single-cell analysis vs Bulk analysis Infographic
 njnir.com
njnir.com