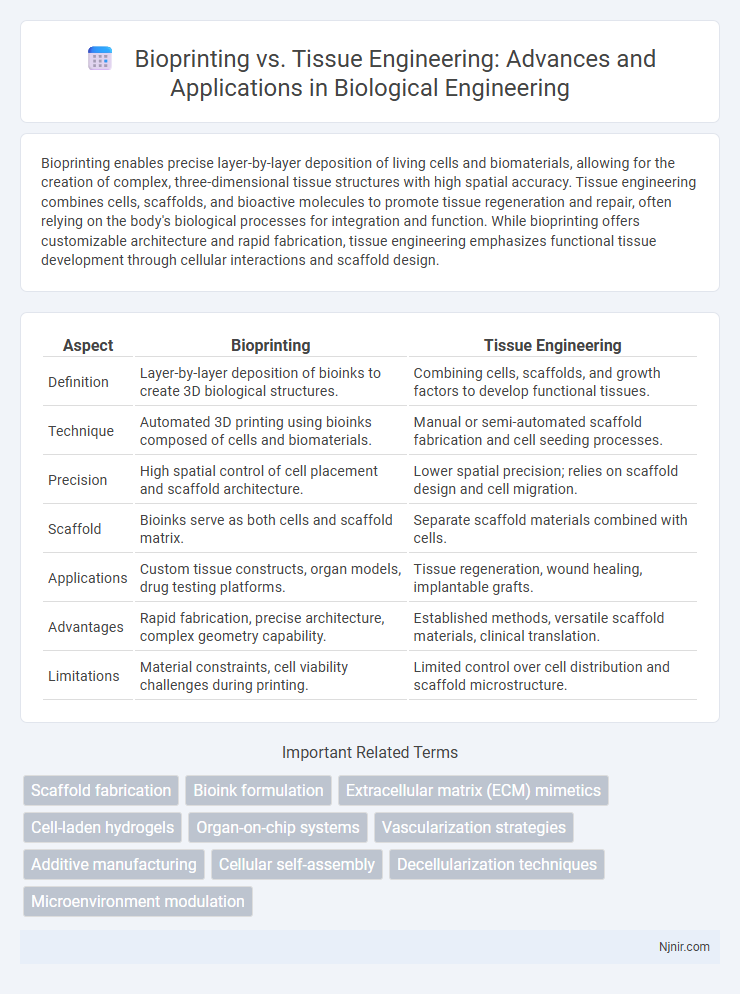
Bioprinting enables precise layer-by-layer deposition of living cells and biomaterials, allowing for the creation of complex, three-dimensional tissue structures with high spatial accuracy. Tissue engineering combines cells, scaffolds, and bioactive molecules to promote tissue regeneration and repair, often relying on the body's biological processes for integration and function. While bioprinting offers customizable architecture and rapid fabrication, tissue engineering emphasizes functional tissue development through cellular interactions and scaffold design.
Table of Comparison
| Aspect | Bioprinting | Tissue Engineering |
|---|---|---|
| Definition | Layer-by-layer deposition of bioinks to create 3D biological structures. | Combining cells, scaffolds, and growth factors to develop functional tissues. |
| Technique | Automated 3D printing using bioinks composed of cells and biomaterials. | Manual or semi-automated scaffold fabrication and cell seeding processes. |
| Precision | High spatial control of cell placement and scaffold architecture. | Lower spatial precision; relies on scaffold design and cell migration. |
| Scaffold | Bioinks serve as both cells and scaffold matrix. | Separate scaffold materials combined with cells. |
| Applications | Custom tissue constructs, organ models, drug testing platforms. | Tissue regeneration, wound healing, implantable grafts. |
| Advantages | Rapid fabrication, precise architecture, complex geometry capability. | Established methods, versatile scaffold materials, clinical translation. |
| Limitations | Material constraints, cell viability challenges during printing. | Limited control over cell distribution and scaffold microstructure. |
Introduction to Bioprinting and Tissue Engineering
Bioprinting utilizes advanced 3D printing technology to precisely deposit living cells and biomaterials layer by layer, enabling the fabrication of complex tissue structures. Tissue engineering combines cells, scaffolds, and bioactive molecules to regenerate or repair damaged tissues through biological and engineering principles. Both fields aim to create functional tissues, but bioprinting offers enhanced spatial control and customization for tissue architecture.
Principles and Techniques of Bioprinting
Bioprinting employs layer-by-layer deposition of bioinks containing living cells and biomaterials guided by computer-aided design to create complex tissue constructs with precise spatial control. Techniques such as inkjet, extrusion, and laser-assisted bioprinting enable the fabrication of vascularized and heterogeneous tissues by maintaining cell viability and functionality during the printing process. This approach contrasts with traditional tissue engineering, which relies on scaffold-based cell seeding and in vitro culture without the direct patterning capability of bioprinting.
Fundamentals of Tissue Engineering
Tissue engineering combines scaffolds, cells, and biologically active molecules to repair or replace damaged tissues, emphasizing cell biology, material science, and biochemistry fundamentals. Bioprinting, an advanced subset, uses 3D printing technology to precisely position cells and biomaterials, enhancing tissue complexity and functionality. Both approaches aim to restore tissue function but differ in fabrication techniques and scalability, with tissue engineering relying more on traditional scaffold-based methods and bioprinting focusing on automated and customizable construction.
Materials Used in Bioprinting vs Tissue Engineering
Bioprinting primarily utilizes bioinks composed of living cells, hydrogels like alginate and collagen, and synthetic polymers tailored for precise 3D printing. Tissue engineering relies on scaffolds made from natural materials such as decellularized extracellular matrix, synthetic biodegradable polymers like polylactic acid (PLA), and composite materials designed to support cell growth and tissue regeneration. Both approaches demand biocompatible and biofunctional materials, but bioprinting emphasizes printability and layer-by-layer deposition, whereas tissue engineering focuses on scaffold architecture and degradation rates.
Advantages and Limitations of Bioprinting
Bioprinting offers precise spatial control over cell placement and scaffold architecture, enabling the creation of complex, patient-specific tissue constructs with improved reproducibility compared to traditional tissue engineering. However, bioprinting faces challenges such as limited bioink options, potential cell damage during the printing process, and difficulties in vascularization of thick tissues. Despite these limitations, bioprinting accelerates tissue fabrication and customization, making it advantageous for regenerative medicine and drug testing applications.
Comparative Analysis: Structural Complexity and Precision
Bioprinting offers superior precision by layering cells and biomaterials with micron-level accuracy, enabling the creation of complex, heterogeneous tissue architectures that closely mimic native tissues. Tissue engineering traditionally relies on scaffold-based methods, which provide structural support but often lack the fine spatial control that bioprinting delivers, limiting the replication of intricate microenvironments. Comparative studies highlight bioprinting's advantage in fabricating vascularized and multicellular structures, enhancing functionality and integration compared to conventional tissue engineering techniques.
Applications in Regenerative Medicine
Bioprinting enables precise fabrication of complex tissue structures by depositing living cells and biomaterials layer-by-layer, enhancing the development of organs and tissue models for regenerative medicine. Tissue engineering focuses on combining scaffolds, cells, and bioactive molecules to repair or replace damaged tissues, with applications in bone regeneration, cartilage repair, and wound healing. Both approaches contribute significantly to personalized medicine by promoting tissue integration, improving functional recovery, and reducing immune rejection risks.
Challenges in Scalability and Clinical Translation
Bioprinting faces significant challenges in scalability due to the intricate control required for cell placement, vascularization, and maintaining cell viability in larger constructs. Tissue engineering struggles with reproducibility and the integration of engineered tissues with host systems, limiting clinical translation. Both fields must overcome issues related to manufacturing consistency, regulatory approval, and long-term functional integration to achieve widespread therapeutic application.
Ethical and Regulatory Considerations
Bioprinting presents unique ethical challenges related to the manipulation of living cells and the potential for creating complex tissues or organs, raising concerns about consent, source of biological materials, and potential misuse. Regulatory frameworks must address the safety, efficacy, and long-term effects of bioprinted tissues, ensuring rigorous testing and quality control while adapting to rapidly evolving technologies. Tissue engineering shares similar ethical and regulatory issues but often involves established methods with clearer guidelines, whereas bioprinting's novelty requires ongoing policy development to balance innovation with public safety.
Future Perspectives in Bioprinting and Tissue Engineering
Future perspectives in bioprinting emphasize advancements in multi-material printing and enhanced cell viability, enabling more complex tissue constructs with precise microarchitecture. Tissue engineering is evolving through the integration of smart biomaterials and gene editing technologies, improving scaffold functionality and promoting regenerative outcomes. Collaboration between bioprinting innovations and tissue engineering strategies holds potential to revolutionize personalized medicine and organ transplantation.
Scaffold fabrication
Bioprinting uses precise layer-by-layer deposition of biomaterials to fabricate complex, customizable scaffolds, whereas traditional tissue engineering relies on manual or mold-based scaffold fabrication methods with limited geometric control.
Bioink formulation
Bioink formulation in bioprinting involves the precise combination of living cells, biomaterials, and growth factors to create structurally and functionally viable tissues, whereas tissue engineering primarily focuses on scaffold design and cell seeding techniques for tissue regeneration.
Extracellular matrix (ECM) mimetics
Bioprinting enables precise spatial deposition of ECM mimetics to replicate native tissue architecture, whereas traditional tissue engineering relies on scaffold-based approaches to integrate ECM components for cellular support.
Cell-laden hydrogels
Cell-laden hydrogels in bioprinting enable precise spatial deposition of living cells for complex tissue constructs, while tissue engineering employs these hydrogels as scaffolds to support cell growth and differentiation in regenerative medicine.
Organ-on-chip systems
Organ-on-chip systems integrate bioprinting techniques and tissue engineering principles to create microfluidic devices that replicate organ-level functions for disease modeling and drug testing.
Vascularization strategies
Bioprinting utilizes precise spatial deposition of cells and biomaterials to create vascular networks, while tissue engineering employs scaffold design and angiogenic factors to promote natural vascularization within engineered tissues.
Additive manufacturing
Additive manufacturing in bioprinting enables precise layer-by-layer deposition of living cells and biomaterials to create complex tissue constructs, whereas traditional tissue engineering relies more on scaffold fabrication and cell seeding without the same level of spatial control.
Cellular self-assembly
Bioprinting leverages precise layer-by-layer cell placement while cellular self-assembly in tissue engineering relies on cells autonomously organizing into functional tissues without external scaffolds.
Decellularization techniques
Decellularization techniques in tissue engineering provide natural extracellular matrix scaffolds that enhance bioprinting by improving cell attachment, proliferation, and tissue regeneration outcomes.
Microenvironment modulation
Bioprinting enhances tissue engineering by precisely modulating the cellular microenvironment through spatial patterning of biomaterials, growth factors, and cells to improve tissue regeneration outcomes.
Bioprinting vs Tissue engineering Infographic
 njnir.com
njnir.com