Epigenome editing modulates gene expression without altering the underlying DNA sequence, offering reversible and precise control over cellular functions. Genome editing directly modifies the DNA sequence, enabling permanent changes that can correct genetic defects but carries higher risks of off-target mutations. Advances in epigenome editing provide safer therapeutic strategies by harnessing endogenous cellular mechanisms to regulate genes dynamically.
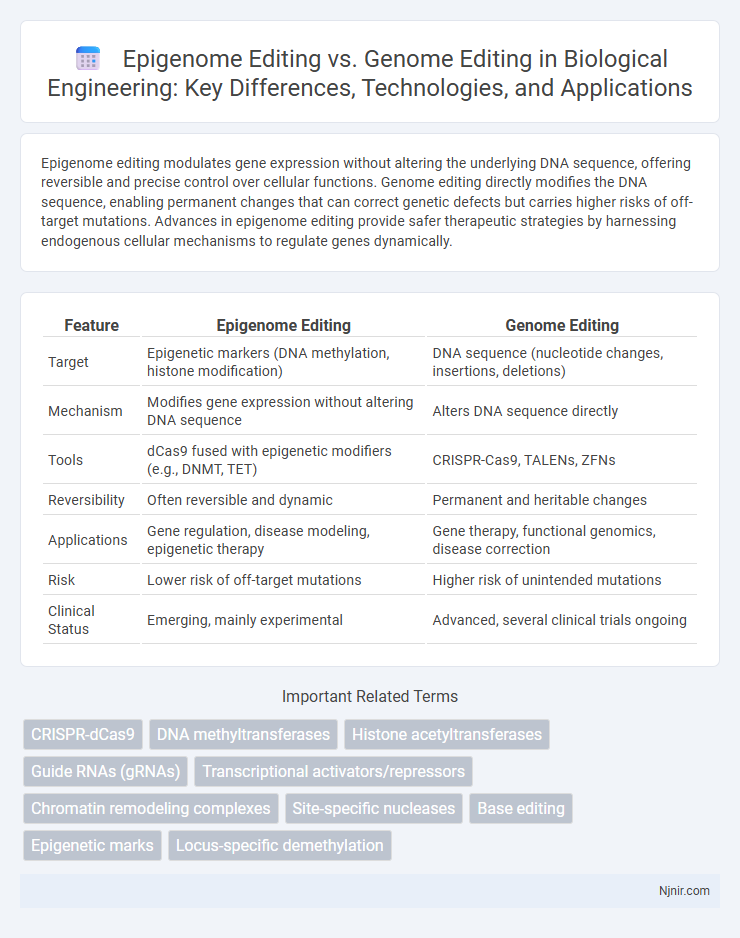
Table of Comparison
| Feature | Epigenome Editing | Genome Editing |
|---|---|---|
| Target | Epigenetic markers (DNA methylation, histone modification) | DNA sequence (nucleotide changes, insertions, deletions) |
| Mechanism | Modifies gene expression without altering DNA sequence | Alters DNA sequence directly |
| Tools | dCas9 fused with epigenetic modifiers (e.g., DNMT, TET) | CRISPR-Cas9, TALENs, ZFNs |
| Reversibility | Often reversible and dynamic | Permanent and heritable changes |
| Applications | Gene regulation, disease modeling, epigenetic therapy | Gene therapy, functional genomics, disease correction |
| Risk | Lower risk of off-target mutations | Higher risk of unintended mutations |
| Clinical Status | Emerging, mainly experimental | Advanced, several clinical trials ongoing |
Introduction to Epigenome and Genome Editing
Epigenome editing involves precise modifications to the chemical markers that regulate gene expression without altering the underlying DNA sequence, enabling reversible control of gene activity. Genome editing, on the other hand, entails targeted changes to the DNA sequence itself, using tools like CRISPR-Cas9 to insert, delete, or replace genetic material. Both techniques offer powerful approaches for studying gene function and developing therapies, with epigenome editing providing an added layer of regulation at the chromatin level.
Underlying Mechanisms: How Genome Editing Works
Genome editing employs engineered nucleases like CRISPR-Cas9, TALENs, or zinc-finger nucleases to introduce double-strand breaks at specific DNA sequences, triggering cellular repair mechanisms such as non-homologous end joining or homology-directed repair to alter the genetic code. These precise alterations enable insertion, deletion, or replacement of DNA segments to modify gene function permanently. In contrast, epigenome editing targets chemical modifications on DNA or histone proteins to regulate gene expression without changing the underlying DNA sequence.
Epigenome Editing: Tools and Techniques
Epigenome editing utilizes tools like CRISPR/dCas9, zinc finger proteins, and TALEs fused with epigenetic modifiers such as DNA methyltransferases or histone acetyltransferases to alter gene expression without changing the DNA sequence. Techniques like targeted DNA methylation/demethylation, histone modification, and chromatin remodeling enable precise control over gene regulation in specific cell types. This reversible and dynamic approach offers advantages over genome editing by minimizing permanent genetic changes and enhancing therapeutic potential in diseases linked to epigenetic dysregulation.
Key Differences Between Epigenome and Genome Editing
Epigenome editing modifies gene expression by altering DNA methylation or histone modifications without changing the underlying DNA sequence, while genome editing directly alters the DNA sequence through technologies like CRISPR-Cas9. Epigenome editing offers reversible and potentially safer therapeutic options by targeting gene regulation mechanisms, whereas genome editing provides permanent changes to genetic code for correcting mutations. The precision of epigenome editing lies in modifying gene function epigenetically, reducing off-target effects linked to permanent DNA alterations common in genome editing.
Applications in Disease Treatment and Therapy
Epigenome editing precisely modifies gene expression without altering the DNA sequence, offering reversible and targeted treatment options for diseases like cancer, neurological disorders, and metabolic conditions. Genome editing involves direct changes to the DNA sequence using tools like CRISPR-Cas9, enabling permanent correction of genetic mutations responsible for inherited diseases such as cystic fibrosis and sickle cell anemia. Both technologies revolutionize personalized medicine, with epigenome editing providing safer modulation of gene activity and genome editing enabling definitive genetic cures.
Precision and Specificity: Comparing Outcomes
Epigenome editing modifies gene expression without altering the underlying DNA sequence, enabling reversible and highly specific regulation at targeted loci, thereby reducing off-target effects compared to genome editing. Genome editing directly alters the DNA sequence, offering permanent changes but with a higher risk of unintended mutations and lower precision at complex genomic regions. Precision in epigenome editing is enhanced by customizable epigenetic modulators, while genome editing relies on engineered nucleases like CRISPR-Cas9 that may cause off-target cleavage, impacting overall specificity and outcomes.
Ethical Considerations and Regulatory Challenges
Epigenome editing offers a reversible and potentially safer approach by modifying gene expression without altering DNA sequences, raising fewer ethical concerns compared to permanent genome editing. Regulatory challenges for epigenome editing involve establishing guidelines for transient modifications and off-target effects, whereas genome editing faces stringent regulations due to heritable changes and potential long-term consequences. Ethical considerations emphasize informed consent, potential for misuse in germline editing, and equitable access, demanding robust frameworks to balance innovation with societal impact.
Current Advances and Innovations
Epigenome editing involves precise modifications to gene expression through reversible changes in DNA methylation and histone modifications without altering the DNA sequence, offering dynamic control of gene function. Recent advances include CRISPR-dCas9 fused with epigenetic effector domains, enabling targeted activation or repression of specific genes linked to diseases like cancer and neurological disorders. Innovations focus on improving delivery systems, minimizing off-target effects, and developing multiplexed edits for complex trait modulation, distinguishing epigenome editing from permanent genome editing technologies such as CRISPR-Cas9 nuclease systems.
Limitations and Potential Risks
Epigenome editing is limited by its transient effects and the challenge of achieving precise, stable modifications without altering the underlying DNA sequence, posing risks of incomplete or reversible therapeutic outcomes. Genome editing, notably using CRISPR-Cas9, faces concerns about off-target mutations, permanent DNA alterations, and potential unintended consequences like insertional mutagenesis or immune responses. Both techniques carry ethical and safety considerations, but the irreversible nature of genome edits contrasts with epigenome editing's reversible, yet less predictable, modifications.
Future Directions in Biological Engineering
Epigenome editing offers reversible and dynamic control over gene expression without altering the underlying DNA sequence, making it a promising alternative to permanent genome editing for therapeutic applications. Advancements in CRISPR-based epigenetic tools enable precise modulation of chromatin states, facilitating targeted gene activation or repression with minimal off-target effects. Future directions in biological engineering emphasize integrating epigenome editing with multi-omics data and artificial intelligence to enhance specificity and develop personalized medicine approaches.
CRISPR-dCas9
CRISPR-dCas9 enables precise epigenome editing by modulating gene expression without altering DNA sequences, contrasting with genome editing that introduces permanent genetic changes.
DNA methyltransferases
Epigenome editing using DNA methyltransferases selectively modifies gene expression by altering DNA methylation patterns without changing the underlying genome sequence, unlike genome editing which directly alters the DNA sequence.
Histone acetyltransferases
Histone acetyltransferases enhance gene expression by modifying chromatin structure in epigenome editing without altering DNA sequences, unlike genome editing which directly changes the DNA code.
Guide RNAs (gRNAs)
Guide RNAs (gRNAs) in epigenome editing precisely target specific DNA regions to modulate gene expression without altering the DNA sequence, whereas in genome editing, gRNAs direct nucleases like CRISPR-Cas9 to induce permanent DNA double-strand breaks for sequence modification.
Transcriptional activators/repressors
Epigenome editing employs transcriptional activators and repressors to reversibly modulate gene expression without altering DNA sequences, whereas genome editing permanently changes DNA sequences through targeted nucleases.
Chromatin remodeling complexes
Epigenome editing utilizes chromatin remodeling complexes to reversibly modify gene expression without altering DNA sequences, whereas genome editing involves permanent DNA sequence changes through targeted nucleases like CRISPR-Cas9.
Site-specific nucleases
Site-specific nucleases enable precise genome editing by directly cutting DNA sequences, whereas epigenome editing modifies gene expression through reversible chemical changes without altering the DNA sequence.
Base editing
Base editing, a precise genome editing technique, modifies specific DNA bases without double-strand breaks, offering targeted genetic corrections while epigenome editing alters gene expression through reversible chemical modifications without changing the DNA sequence.
Epigenetic marks
Epigenome editing precisely modifies epigenetic marks such as DNA methylation and histone modifications to regulate gene expression without altering the underlying DNA sequence, unlike genome editing which directly changes the DNA sequence.
Locus-specific demethylation
Locus-specific demethylation in epigenome editing offers precise, reversible regulation of gene expression without altering DNA sequence, unlike genome editing which induces permanent DNA modifications.
Epigenome editing vs Genome editing Infographic
 njnir.com
njnir.com