Microfluidics offers precise control over cellular microenvironments, enabling high-throughput screening and reduced reagent volumes compared to macroscale bioreactors. These microscale systems facilitate rapid manipulation of fluids at the micron level, enhancing cell culture uniformity and experimental reproducibility. Macroscale bioreactors, while providing larger volumes for industrial-scale production, often lack the fine spatial and temporal resolution achievable with microfluidic platforms.
Table of Comparison
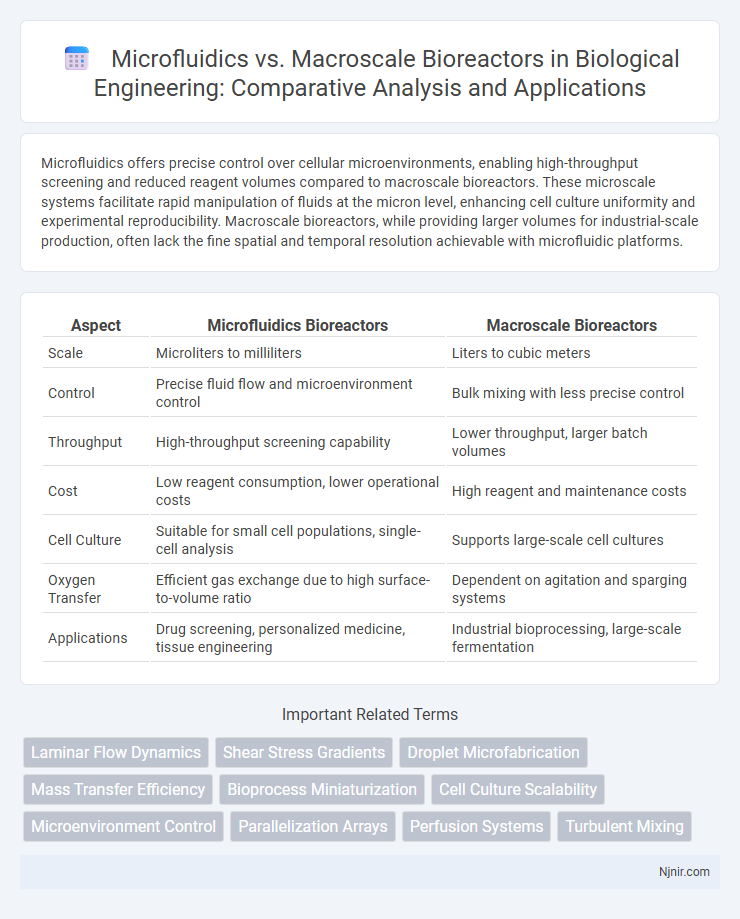
| Aspect | Microfluidics Bioreactors | Macroscale Bioreactors |
|---|---|---|
| Scale | Microliters to milliliters | Liters to cubic meters |
| Control | Precise fluid flow and microenvironment control | Bulk mixing with less precise control |
| Throughput | High-throughput screening capability | Lower throughput, larger batch volumes |
| Cost | Low reagent consumption, lower operational costs | High reagent and maintenance costs |
| Cell Culture | Suitable for small cell populations, single-cell analysis | Supports large-scale cell cultures |
| Oxygen Transfer | Efficient gas exchange due to high surface-to-volume ratio | Dependent on agitation and sparging systems |
| Applications | Drug screening, personalized medicine, tissue engineering | Industrial bioprocessing, large-scale fermentation |
Introduction to Microfluidics and Macroscale Bioreactors
Microfluidics involves the manipulation of fluids at the microscale, enabling precise control over reaction environments, which enhances cell culture and biochemical analysis through reduced reagent volumes and improved mass transfer. Macroscale bioreactors operate on a larger scale, supporting high-volume production processes with robust mixing, aeration, and sensor integration for industrial bioprocesses. The comparison highlights microfluidics' advantages in experimental efficiency and scalability challenges faced by macroscale bioreactors in maintaining uniform conditions throughout large volumes.
Fundamental Principles and Design Differences
Microfluidics relies on laminar flow and precise control of fluid dynamics within microscale channels, enabling high surface-to-volume ratios and rapid mass transfer, contrasting macroscale bioreactors that operate under turbulent flow with larger volumes. Design differences include the use of microfabricated materials and integrated sensors in microfluidic devices for real-time monitoring, whereas macroscale bioreactors utilize stirred tanks, spargers, and large impellers to ensure homogenous mixing and aeration. Fundamental principles emphasize microfluidics' reliance on diffusion-dominated transport, while macroscale bioreactors depend on convection-driven mixing and scaling strategies to maintain cell culture conditions.
Fluid Dynamics: Micro vs. Macro Environments
Microfluidics enables precise control of fluid flow at the microscale, where laminar flow dominates and diffusion is the primary mode of mass transport, resulting in enhanced nutrient delivery and waste removal for cell cultures. In contrast, macroscale bioreactors rely on turbulent flow and mechanical stirring to achieve mixing and oxygenation, which can create heterogeneous environments and shear stress impacting cell viability. The distinct fluid dynamics between micro and macro environments significantly influence bioprocess efficiency, scalability, and cell behavior outcomes.
Scalability and Throughput Considerations
Microfluidics offers precise control over cellular environments with high-throughput screening capabilities but faces challenges in scaling up to volumes necessary for industrial bioprocesses. Macroscale bioreactors provide robust scalability to large production volumes but often lack the fine control and rapid experiment turnaround characteristic of microfluidic systems. Balancing the scalability of macroscale bioreactors with the throughput efficiency of microfluidics remains critical for optimizing biomanufacturing processes.
Cellular Behavior and Biomimicry in Each System
Microfluidics enables precise control over cellular microenvironments, allowing for enhanced mimicry of in vivo conditions and more accurate studies of cell behavior such as migration, differentiation, and response to stimuli. Macroscale bioreactors support larger cell populations and bulk tissue-like structures but often lack the fine spatial and temporal control necessary for replicating complex tissue-specific microenvironments. Biomimicry in microfluidic systems excels through dynamic gradients and shear stress modulation, whereas macroscale bioreactors better simulate overall biochemical and mechanical cues relevant to organ-level functions.
Analytical Sensitivity and Data Acquisition
Microfluidics offers significantly higher analytical sensitivity compared to macroscale bioreactors due to reduced sample volumes and enhanced control over microenvironmental conditions, enabling precise single-cell or molecular-level analysis. The compact design of microfluidic systems facilitates rapid, real-time data acquisition with high temporal and spatial resolution, surpassing the slower, bulk measurements typical in macroscale bioreactors. Enhanced integration of sensors and automation in microfluidics further streamlines data collection and processing, providing richer datasets crucial for detailed biological insights and optimization.
Integration with Biosensors and Monitoring Technologies
Microfluidics offers superior integration with biosensors and real-time monitoring technologies due to its precise control of small volumes and rapid response times, enabling high-throughput and dynamic analysis of cellular responses. In contrast, macroscale bioreactors rely on bulk measurements and often require external sampling, resulting in delayed data acquisition and less spatial resolution. The microfluidic platform's compatibility with advanced electrochemical and optical biosensors facilitates continuous, non-invasive monitoring of key metabolites, pH, and oxygen levels, outperforming traditional bioreactor systems in process optimization and control.
Cost, Accessibility, and Manufacturing Challenges
Microfluidics offers lower operational costs and increased accessibility due to its minimal reagent consumption and compact design, making it ideal for high-throughput screening and lab-scale experiments. Macroscale bioreactors involve higher capital investment, complex manufacturing, and maintenance challenges but provide scalability essential for industrial bioprocessing. Manufacturing challenges for microfluidics include precise microfabrication and integration of sensors, whereas macroscale bioreactors require robust materials and systems for mixing, mass transfer, and sterility.
Applications in Tissue Engineering and Bioprocessing
Microfluidics offers precise control over cellular microenvironments, enabling high-throughput screening and mimicking physiological conditions essential for tissue engineering. Macroscale bioreactors support large-volume cell culture and scalable bioprocessing, facilitating mass production of biological products such as vaccines and monoclonal antibodies. Integration of microfluidic systems with macroscale bioreactors enhances tissue construct maturation and optimizes bioprocess efficiency through improved nutrient delivery and dynamic monitoring.
Future Trends and Hybrid Platform Innovations
Future trends in microfluidics emphasize enhanced precision, scalability, and integration with real-time sensing technologies to overcome limitations of traditional macroscale bioreactors. Hybrid platform innovations combine microfluidic devices with macroscale bioreactors to optimize cell culture conditions, improve mass transfer, and enable high-throughput screening while maintaining industrial production capacity. Advances in materials, automation, and artificial intelligence-driven control systems are driving the development of hybrid bioreactor platforms that offer superior efficiency, reproducibility, and scalability for biomanufacturing applications.
Laminar Flow Dynamics
Microfluidics enables precise control of laminar flow dynamics at microscale channels, enhancing nutrient mixing and cellular interactions, whereas macroscale bioreactors exhibit turbulent flow that impacts mass transfer efficiency and shear stress on cells.
Shear Stress Gradients
Microfluidics offer precise control of shear stress gradients with micrometer-scale resolution, enabling more biomimetic environments compared to the heterogeneous and higher shear stress gradients in macroscale bioreactors.
Droplet Microfabrication
Droplet microfabrication in microfluidics enables precise control over cell encapsulation and reaction environments, offering higher throughput and reduced reagent consumption compared to traditional macroscale bioreactors.
Mass Transfer Efficiency
Microfluidic bioreactors exhibit significantly higher mass transfer efficiency than macroscale bioreactors due to their enhanced surface-to-volume ratio and precise flow control.
Bioprocess Miniaturization
Microfluidics enables precise control and high-throughput screening in bioprocess miniaturization, significantly reducing reagent use and accelerating optimization compared to traditional macroscale bioreactors.
Cell Culture Scalability
Microfluidics enables precise control and high-throughput scalability of cell cultures at microscale volumes, whereas macroscale bioreactors support large-scale production with robust mixing and nutrient delivery but face challenges in replicating microenvironmental conditions.
Microenvironment Control
Microfluidic bioreactors offer precise microenvironment control through enhanced spatial and temporal regulation of shear stress, nutrient gradients, and cellular interactions compared to macroscale bioreactors.
Parallelization Arrays
Parallelization arrays in microfluidics enable high-throughput, precise control and scalability surpassing macroscale bioreactors by allowing simultaneous, independent experiments with minimal reagent consumption.
Perfusion Systems
Perfusion systems in microfluidics enable precise, continuous nutrient and waste exchange at the cellular level, outperforming macroscale bioreactors by enhancing cell culture efficiency and scalability.
Turbulent Mixing
Microfluidics offers enhanced control over laminar flow and precise mixing at microscale, whereas macroscale bioreactors rely on turbulent mixing to achieve homogeneity in larger volumes.
Microfluidics vs Macroscale bioreactors Infographic
 njnir.com
njnir.com