Dosimetry measures the absorbed dose of ionizing radiation in materials or tissues, crucial for assessing radiation exposure and ensuring safety in nuclear engineering environments. Spectrometry analyzes the energy spectrum of radiation to identify radioactive isotopes and characterize radiation sources, enabling precise monitoring and control of nuclear processes. Both techniques complement each other by providing quantitative dose assessment and qualitative radiation profiling for effective radiation management.
Table of Comparison
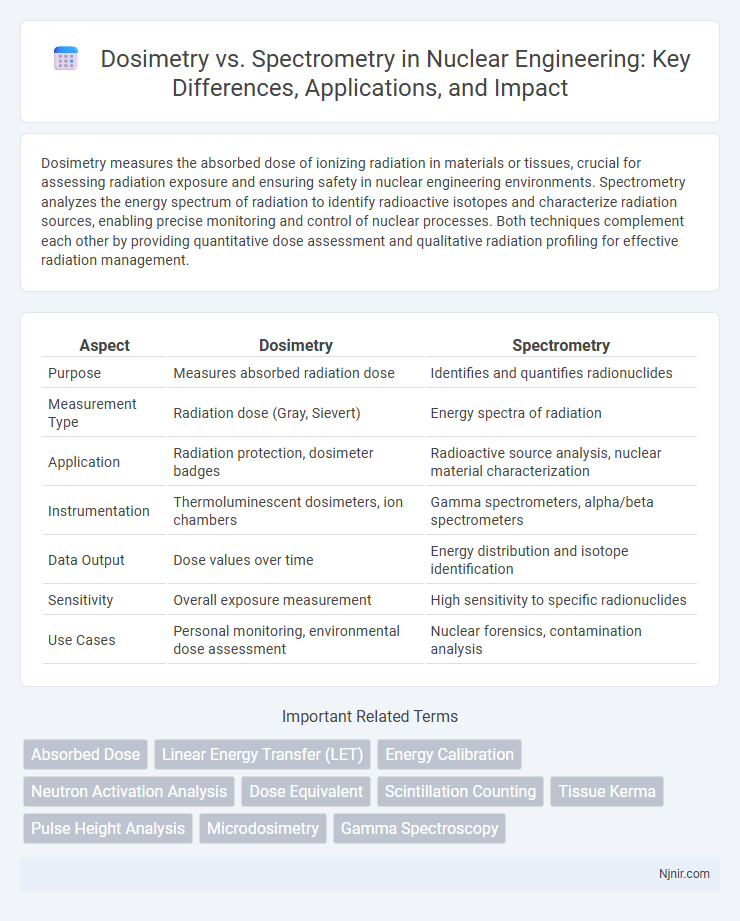
| Aspect | Dosimetry | Spectrometry |
|---|---|---|
| Purpose | Measures absorbed radiation dose | Identifies and quantifies radionuclides |
| Measurement Type | Radiation dose (Gray, Sievert) | Energy spectra of radiation |
| Application | Radiation protection, dosimeter badges | Radioactive source analysis, nuclear material characterization |
| Instrumentation | Thermoluminescent dosimeters, ion chambers | Gamma spectrometers, alpha/beta spectrometers |
| Data Output | Dose values over time | Energy distribution and isotope identification |
| Sensitivity | Overall exposure measurement | High sensitivity to specific radionuclides |
| Use Cases | Personal monitoring, environmental dose assessment | Nuclear forensics, contamination analysis |
Introduction to Dosimetry and Spectrometry
Dosimetry quantifies radiation dose absorbed by matter, essential for ensuring safety in medical, industrial, and environmental applications. Spectrometry analyzes the energy spectrum of radiation, enabling identification and characterization of radioactive materials. Understanding the principles of dosimetry and spectrometry is crucial for accurate radiation measurement and protection.
Fundamental Principles of Dosimetry
Dosimetry measures the absorbed dose of ionizing radiation in matter, using principles based on energy deposition per unit mass, often quantified in grays (Gy). It relies on detecting the interaction of radiation with a medium to calculate the dose, crucial for radiation therapy and protection. Spectrometry, by contrast, identifies and quantifies the energy spectra of radiation, serving as a tool for characterizing radiation types rather than directly measuring dose.
Core Concepts in Spectrometry
Spectrometry centers on measuring the interaction of electromagnetic radiation with matter to analyze chemical composition and structural properties, utilizing core concepts such as wavelength, frequency, and intensity. It employs techniques like mass spectrometry, atomic absorption, and infrared spectrometry to detect and quantify elements or compounds based on their spectral signatures. Unlike dosimetry, which measures absorbed radiation doses primarily for safety and medical applications, spectrometry emphasizes detailed molecular and atomic characterization through precise spectral data.
Key Differences between Dosimetry and Spectrometry
Dosimetry measures the absorbed dose of ionizing radiation in materials or biological tissues, quantifying radiation exposure primarily for safety and health monitoring. Spectrometry analyzes the energy spectrum of electromagnetic radiation or particles to identify and quantify the composition of substances, using instruments like mass spectrometers or gamma spectrometers. Key differences include their objectives--dosimetry focuses on dose measurement for radiation protection, while spectrometry concentrates on spectral analysis for material characterization and identification.
Applications of Dosimetry in Nuclear Engineering
Dosimetry in nuclear engineering is crucial for accurately measuring radiation doses to ensure safety in reactor operations, radiation shielding design, and waste management. It provides essential data for monitoring exposure levels of personnel and environments to prevent radiation hazards and comply with regulatory standards. Spectrometry, while primarily used for identifying and quantifying radionuclides, complements dosimetry by characterizing radiation sources for effective dose assessment and control measures.
Uses of Spectrometry in Nuclear Facilities
Spectrometry in nuclear facilities plays a crucial role in identifying and quantifying radioactive isotopes, enhancing safety through precise radiation analysis. It enables real-time monitoring of radiation types and energy levels, essential for controlling nuclear reactions and preventing contamination. This method supports regulatory compliance by providing detailed spectral data for environmental monitoring and waste management.
Instrumentation for Dosimetry and Spectrometry
Dosimetry instrumentation primarily includes ionization chambers, thermoluminescent dosimeters (TLDs), and solid-state detectors designed to measure the absorbed dose of radiation in a medium, ensuring precise dose quantification for radiation therapy and safety monitoring. Spectrometry instrumentation involves the use of scintillation detectors, semiconductor detectors, and multichannel analyzers to identify and quantify the energy spectrum of radiation, enabling detailed analysis of radiation sources and isotopes. Both instrument types integrate digital electronics and data acquisition systems to enhance measurement accuracy, but dosimeters emphasize dose measurement while spectrometers focus on spectral resolution and energy discrimination.
Calibration and Quality Assurance Procedures
Dosimetry calibration involves precise measurement of absorbed radiation dose using standard radiation sources to ensure accurate and consistent readings in medical and industrial applications. Spectrometry calibration requires the use of known spectral lines or reference materials to adjust the instrument's response for accurate identification and quantification of elements or isotopes. Quality assurance procedures for dosimetry include routine checks with phantom materials and dose meters, while spectrometry quality control relies on periodic validation with certified reference standards and instrument performance tests.
Challenges and Limitations
Dosimetry faces challenges in accurately quantifying dose distribution due to tissue heterogeneity and energy dependence of detectors, limiting precise patient dose assessment. Spectrometry struggles with spectral overlap and detector resolution constraints, complicating the identification of specific radionuclides or chemical species in complex samples. Both techniques require sophisticated calibration and correction methods to mitigate measurement uncertainties and enhance reliability.
Future Trends in Dosimetry and Spectrometry
Future trends in dosimetry emphasize the integration of advanced nanomaterials and real-time wireless monitoring systems for enhanced radiation dose accuracy and patient safety. Spectrometry advancements focus on high-resolution mass spectrometers and machine learning algorithms to improve the detection sensitivity and data analysis of complex chemical compositions. The convergence of miniaturized devices and AI-driven data processing is poised to revolutionize both dosimetry and spectrometry for medical, environmental, and industrial applications.
Absorbed Dose
Absorbed Dose, a key metric in dosimetry, quantifies the energy deposited by ionizing radiation per unit mass of tissue, while spectrometry primarily analyzes the energy spectra of radiation without directly measuring dose absorption.
Linear Energy Transfer (LET)
Linear Energy Transfer (LET) is a critical parameter in dosimetry for quantifying energy deposition in tissues, while spectrometry analyzes radiation energy spectra without directly measuring LET values.
Energy Calibration
Energy calibration in dosimetry ensures accurate measurement of absorbed radiation doses, while in spectrometry it precisely identifies photon or particle energies for detailed spectral analysis.
Neutron Activation Analysis
Neutron Activation Analysis combines dosimetry to measure radiation dose with spectrometry techniques to identify and quantify elements based on neutron-induced radioactivity.
Dose Equivalent
Dose equivalent in dosimetry quantifies biological effects of radiation by weighting absorbed dose with radiation quality factors, whereas spectrometry precisely measures energy spectra of radiation without directly assessing biological impact.
Scintillation Counting
Scintillation counting in dosimetry measures radiation dose by detecting emitted light from a scintillator, while in spectrometry, it analyzes energy spectra of radiation for isotope identification.
Tissue Kerma
Tissue Kerma quantifies the kinetic energy released per unit mass of tissue and serves as a critical parameter in dosimetry to assess radiation dose, while spectrometry analyzes energy spectra of radiation for qualitative and quantitative characterization.
Pulse Height Analysis
Pulse Height Analysis enhances spectrometry by precisely measuring energy deposition in dosimetry, enabling accurate radiation dose assessment and material identification.
Microdosimetry
Microdosimetry precisely measures energy deposition at microscopic scales to assess radiation effects, unlike spectrometry which primarily analyzes radiation energy spectra without spatial resolution.
Gamma Spectroscopy
Gamma spectroscopy provides precise isotopic identification and quantification by analyzing gamma-ray energy spectra, while dosimetry measures the absorbed radiation dose to assess exposure effects.
dosimetry vs spectrometry Infographic
 njnir.com
njnir.com