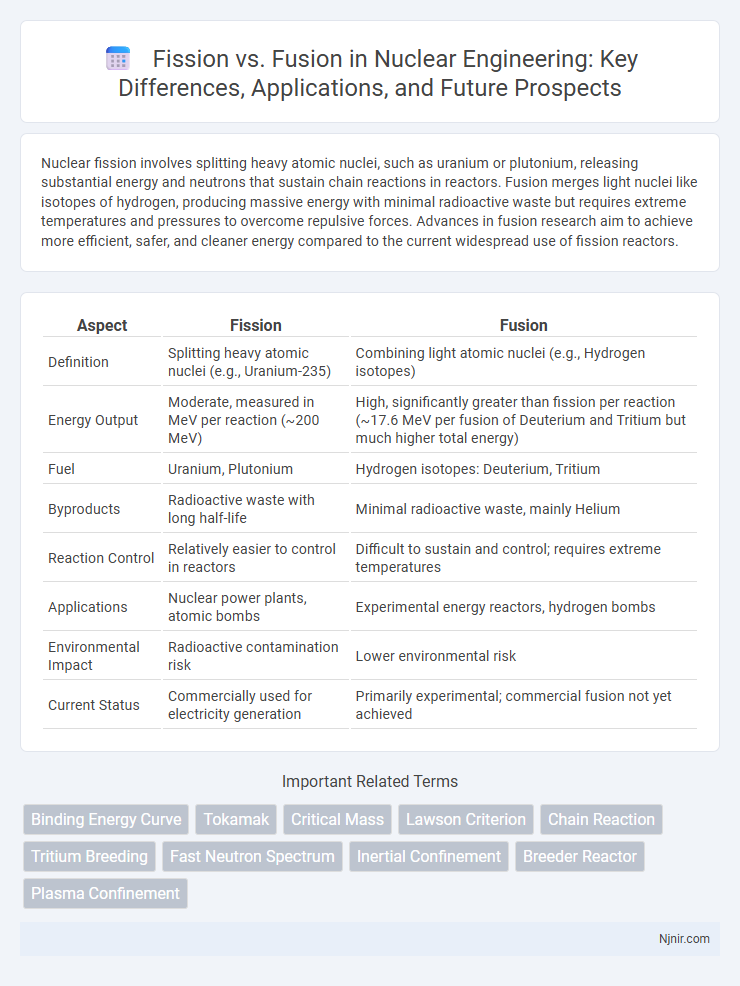
Nuclear fission involves splitting heavy atomic nuclei, such as uranium or plutonium, releasing substantial energy and neutrons that sustain chain reactions in reactors. Fusion merges light nuclei like isotopes of hydrogen, producing massive energy with minimal radioactive waste but requires extreme temperatures and pressures to overcome repulsive forces. Advances in fusion research aim to achieve more efficient, safer, and cleaner energy compared to the current widespread use of fission reactors.
Table of Comparison
| Aspect | Fission | Fusion |
|---|---|---|
| Definition | Splitting heavy atomic nuclei (e.g., Uranium-235) | Combining light atomic nuclei (e.g., Hydrogen isotopes) |
| Energy Output | Moderate, measured in MeV per reaction (~200 MeV) | High, significantly greater than fission per reaction (~17.6 MeV per fusion of Deuterium and Tritium but much higher total energy) |
| Fuel | Uranium, Plutonium | Hydrogen isotopes: Deuterium, Tritium |
| Byproducts | Radioactive waste with long half-life | Minimal radioactive waste, mainly Helium |
| Reaction Control | Relatively easier to control in reactors | Difficult to sustain and control; requires extreme temperatures |
| Applications | Nuclear power plants, atomic bombs | Experimental energy reactors, hydrogen bombs |
| Environmental Impact | Radioactive contamination risk | Lower environmental risk |
| Current Status | Commercially used for electricity generation | Primarily experimental; commercial fusion not yet achieved |
Introduction to Nuclear Reactions
Nuclear reactions involve changes in an atom's nucleus that release significant energy, primarily through fission or fusion processes. Fission splits heavy atomic nuclei such as uranium-235 or plutonium-239 into smaller fragments, producing energy, neutrons, and radioactive byproducts. Fusion combines light nuclei like hydrogen isotopes deuterium and tritium to form a heavier nucleus, releasing vast amounts of energy with minimal radioactive waste.
Fundamental Principles of Fission
Nuclear fission involves the splitting of a heavy atomic nucleus, such as uranium-235 or plutonium-239, into smaller nuclei, releasing a significant amount of energy through the conversion of mass to energy as described by Einstein's equation, E=mc2. This process generates free neutrons that can induce further fission reactions, creating a controlled or uncontrolled chain reaction. Fission's fundamental principle relies on the instability of large nuclei and the energy released when the strong nuclear force holding the nucleus together is overcome.
Fundamental Principles of Fusion
Fusion involves combining light atomic nuclei, such as hydrogen isotopes deuterium and tritium, at extremely high temperatures and pressures to form a heavier nucleus, releasing vast amounts of energy due to mass-to-energy conversion governed by Einstein's equation, E=mc2. This process mimics the sun's core reactions and requires overcoming the electrostatic repulsion between positively charged nuclei, a challenge met through creating plasma states at tens of millions of degrees kelvin. Fusion's fundamental principle lies in achieving and sustaining these extreme conditions to enable nuclear reactions that produce clean energy with minimal radioactive waste compared to fission.
Energy Output Comparison
Nuclear fusion produces significantly more energy than fission, with fusion reactions releasing energy equivalent to millions of times more than chemical reactions per unit mass. Fusion of light nuclei such as hydrogen isotopes yields energy up to four times greater per reaction than typical fission of heavy elements like uranium or plutonium. The energy density of fusion fuels surpasses fission fuels, making fusion a more efficient source of power despite current technological challenges in sustaining controlled fusion reactions.
Fuel Sources and Availability
Fission relies primarily on uranium-235 and plutonium-239, which are finite and mined from specific geographic locations, limiting availability and raising concerns about long-term sustainability. Fusion uses isotopes of hydrogen, such as deuterium and tritium, abundant in seawater, offering a virtually limitless fuel supply. The widespread availability of fusion fuels positions it as a more sustainable energy source compared to the limited and geopolitically constrained fission fuels.
Safety and Risk Analysis
Nuclear fission involves splitting heavy atomic nuclei, producing radioactive waste and a higher risk of meltdown incidents, as seen in historical accidents like Chernobyl and Fukushima. In contrast, nuclear fusion, which merges light atomic nuclei, has inherently safer fuel cycles with minimal long-lived radioactive waste and a significantly lower risk of catastrophic failure due to its reliance on plasma confinement at extreme temperatures. Safety analyses highlight fusion's potential for passive safety features, such as self-terminating reactions, making it a promising alternative to fission in reducing long-term environmental and human health risks.
Waste Generation and Management
Nuclear fission produces a significant amount of long-lived radioactive waste, requiring complex, secure storage solutions such as deep geological repositories to prevent environmental contamination. Fusion generates substantially less radioactive waste, primarily from neutron activation of reactor materials, resulting in shorter-lived and more manageable byproducts. The reduced waste production and simpler waste management challenges make fusion a more environmentally sustainable option compared to fission.
Technological Challenges
Fission technology faces challenges such as managing radioactive waste, ensuring reactor safety, and controlling nuclear reactions to prevent meltdowns. Fusion technology requires overcoming extreme temperature containment, achieving sustained plasma stability, and developing materials that can endure intense neutron bombardment. Both processes demand advancements in engineering precision, energy efficiency, and scalable reactor designs to become viable energy sources.
Current and Future Applications
Nuclear fission powers about 10% of the world's electricity by splitting heavy atomic nuclei, widely used in existing nuclear reactors for stable energy supply and naval propulsion. Fusion, which merges light nuclei like hydrogen isotopes, holds potential for nearly limitless clean energy with ongoing projects such as ITER aiming to achieve net energy gain. Future applications of fusion include commercial power plants with minimal radioactive waste, hydrogen production, and space propulsion, promising a transformative impact on global energy systems.
Environmental Impact and Sustainability
Nuclear fusion produces minimal long-lived radioactive waste compared to nuclear fission, significantly reducing environmental hazards and waste management challenges. Fusion fuel, primarily isotopes of hydrogen like deuterium and tritium, is abundant and can provide a nearly limitless energy supply without the carbon emissions associated with fossil fuels. Fission reactors generate high-level radioactive waste and pose risks of nuclear accidents, making fusion a more sustainable and environmentally friendly energy source for the future.
Binding Energy Curve
The Binding Energy Curve demonstrates that nuclear fusion releases energy by combining light nuclei with higher binding energy per nucleon, while fission releases energy by splitting heavy nuclei with lower binding energy per nucleon.
Tokamak
Tokamak reactors utilize magnetic confinement to achieve fusion by heating hydrogen isotopes to millions of degrees, enabling the fusion of nuclei and release of immense energy, unlike fission which splits heavy atoms to produce power.
Critical Mass
Critical mass in fission is the minimum amount of fissile material needed to sustain a chain reaction, whereas fusion requires extremely high temperatures and pressures to overcome electrostatic forces without a defined critical mass.
Lawson Criterion
Fusion achieves net energy gain by surpassing the Lawson Criterion, which defines the minimum product of plasma density, temperature, and confinement time, whereas fission relies on nuclear chain reactions without this specific threshold.
Chain Reaction
Fission relies on a chain reaction where splitting heavy atomic nuclei releases neutrons that trigger further splits, while fusion requires extreme conditions to merge light nuclei without a self-sustaining chain reaction.
Tritium Breeding
Tritium breeding in fusion reactors relies on lithium-containing blankets to produce tritium via neutron interactions, addressing fuel scarcity challenges absent in fission reactors that utilize uranium or plutonium fission.
Fast Neutron Spectrum
Fast neutron spectrum in fission reactors enables efficient fuel utilization and waste reduction, while fusion relies on high-energy neutron generation for potential clean energy without long-lived radioactive waste.
Inertial Confinement
Inertial confinement fusion uses intense laser or ion beams to compress and heat fuel pellets, enabling fusion reactions by overcoming the limitations of fission's chain reactions.
Breeder Reactor
Breeder reactors enhance nuclear fuel efficiency by converting fertile isotopes like U-238 into fissile material through fission, contrasting with fusion's process of combining light nuclei to release energy.
Plasma Confinement
Magnetic confinement in fusion reactors uses strong magnetic fields to contain high-temperature plasma, whereas fission relies on solid fuel rods without plasma confinement, highlighting the critical role of plasma confinement in achieving sustainable nuclear fusion reactions.
Fission vs Fusion Infographic
 njnir.com
njnir.com