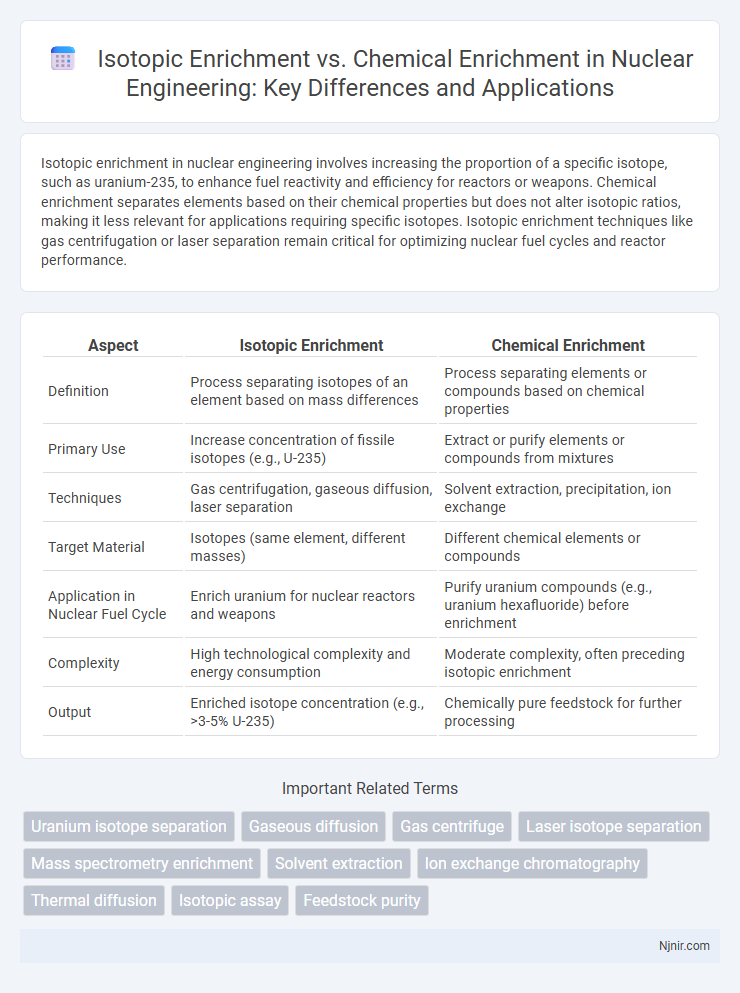
Isotopic enrichment in nuclear engineering involves increasing the proportion of a specific isotope, such as uranium-235, to enhance fuel reactivity and efficiency for reactors or weapons. Chemical enrichment separates elements based on their chemical properties but does not alter isotopic ratios, making it less relevant for applications requiring specific isotopes. Isotopic enrichment techniques like gas centrifugation or laser separation remain critical for optimizing nuclear fuel cycles and reactor performance.
Table of Comparison
| Aspect | Isotopic Enrichment | Chemical Enrichment |
|---|---|---|
| Definition | Process separating isotopes of an element based on mass differences | Process separating elements or compounds based on chemical properties |
| Primary Use | Increase concentration of fissile isotopes (e.g., U-235) | Extract or purify elements or compounds from mixtures |
| Techniques | Gas centrifugation, gaseous diffusion, laser separation | Solvent extraction, precipitation, ion exchange |
| Target Material | Isotopes (same element, different masses) | Different chemical elements or compounds |
| Application in Nuclear Fuel Cycle | Enrich uranium for nuclear reactors and weapons | Purify uranium compounds (e.g., uranium hexafluoride) before enrichment |
| Complexity | High technological complexity and energy consumption | Moderate complexity, often preceding isotopic enrichment |
| Output | Enriched isotope concentration (e.g., >3-5% U-235) | Chemically pure feedstock for further processing |
Introduction to Isotopic and Chemical Enrichment
Isotopic enrichment involves increasing the concentration of a specific isotope within a sample, commonly used in nuclear fuel preparation and medical diagnostics to enhance target isotope availability. Chemical enrichment refers to separating substances based on their chemical properties and affinities, often applied in industrial processes like uranium extraction and drug purification. Understanding these methods is crucial for advancing applications in energy production, healthcare, and material sciences.
Fundamental Principles of Isotopic Enrichment
Isotopic enrichment involves increasing the proportion of a specific isotope in a mixture based on differences in atomic mass or nuclear properties, primarily utilizing physical methods such as centrifugation, diffusion, or laser separation. Chemical enrichment relies on the subtle variations in chemical reaction rates or equilibrium constants between isotopes, exploiting isotopic fractionation during chemical processes. Fundamental principles highlight that isotopic enrichment exploits mass-dependent physical separation, while chemical enrichment is governed by isotope-specific chemical kinetics and thermodynamics.
Core Concepts of Chemical Enrichment
Chemical enrichment involves altering the elemental composition of a substance through chemical reactions, often targeting specific isotopes within compounds by exploiting differences in chemical behavior. This process contrasts with isotopic enrichment, which separates isotopes based on physical properties such as mass or diffusion rates rather than chemical affinity. Core concepts of chemical enrichment include selective chemical reactions, isotope-specific bonding affinities, and tailored reagents that facilitate preferential isotope incorporation or separation.
Key Techniques for Isotopic Separation
Key techniques for isotopic separation include gas centrifugation, electromagnetic isotope separation, and laser isotope separation, each exploiting mass differences between isotopes to achieve enrichment. Gas centrifugation uses high-speed rotation to separate isotopes based on mass, commonly applied in uranium enrichment. Laser isotope separation employs precise photon energies to selectively excite and separate specific isotopes, offering high efficiency and specificity compared to chemical enrichment methods that rely on isotopic chemical reactivity differences.
Major Methods Used in Chemical Enrichment
Major methods used in chemical enrichment include liquid-liquid extraction, ion exchange, and chemical precipitation, each exploiting differences in chemical properties to separate isotopes effectively. Liquid-liquid extraction leverages preferential solubility of isotopes in two immiscible liquids, while ion exchange utilizes resin beads to selectively adsorb specific isotopic ions from a solution. Chemical precipitation involves converting isotopes into insoluble compounds, allowing selective separation based on varying solubility and reaction rates.
Comparative Efficiency: Isotopic vs Chemical Processes
Isotopic enrichment achieves higher separation factors by exploiting mass differences between isotopes, making it more efficient for producing highly pure isotopes compared to chemical enrichment, which relies on slight variations in chemical properties. Chemical enrichment processes often require multiple stages and longer processing times due to lower selectivity, whereas isotopic methods such as centrifugation or laser-based separation provide faster and more precise concentration of target isotopes. The energy consumption and operational complexity of isotopic enrichment can be higher, but it yields superior purity levels critical for nuclear, medical, and scientific applications.
Industrial Applications and Use Cases
Isotopic enrichment is crucial in nuclear energy production, where uranium-235 is separated from uranium-238 to create fuel for reactors, enabling efficient fission processes. Chemical enrichment, involving selective chemical reactions or separations, is applied in pharmaceutical manufacturing to produce isotopically labeled compounds for drug development and metabolic studies. Industrial applications also include semiconductor fabrication, where isotopic purification improves material properties such as thermal conductivity and electron mobility, enhancing device performance.
Safety and Environmental Implications
Isotopic enrichment methods, such as gas centrifugation and laser separation, pose fewer chemical hazards but require stringent radiation shielding and containment to mitigate nuclear risks. Chemical enrichment processes often involve hazardous reagents and generate toxic waste, increasing environmental contamination and worker safety concerns. Effective management of both methods demands robust safety protocols and waste treatment systems to minimize ecological impact and human exposure.
Economic Considerations and Cost Analysis
Isotopic enrichment typically involves advanced centrifuge or laser-based technologies that demand significant capital investment and high operational costs due to energy consumption and specialized equipment. Chemical enrichment methods often incur lower initial costs but can experience higher long-term expenses related to chemicals, waste management, and slower process throughput. Economic considerations balance the scalability and precision of isotopic enrichment against the simpler setup and potentially greater environmental liabilities of chemical enrichment.
Future Trends in Nuclear Material Enrichment
Future trends in nuclear material enrichment emphasize advanced isotopic enrichment techniques such as laser-based methods and gas centrifuge technology to achieve higher precision and efficiency in separating fissile isotopes like U-235. Chemical enrichment processes are increasingly supplemented or replaced by physical methods that minimize chemical waste and enhance proliferation resistance. Innovations in molecular laser isotope separation (MLIS) and aerodynamic processes are poised to lower operational costs while improving scalability for next-generation nuclear fuel cycles.
Uranium isotope separation
Isotopic enrichment of uranium separates U-235 from U-238 using physical methods like gas centrifuges, whereas chemical enrichment relies on differences in chemical reactions but is less effective for uranium isotope separation.
Gaseous diffusion
Gaseous diffusion, a method of isotopic enrichment, separates uranium isotopes based on slight differences in molecular velocities through a porous membrane, offering higher precision compared to chemical enrichment techniques that rely on chemical properties.
Gas centrifuge
Gas centrifuge technology achieves isotopic enrichment by exploiting centrifugal force to separate isotopes based on mass differences, whereas chemical enrichment relies on selective chemical reactions to isolate specific isotopes.
Laser isotope separation
Laser isotope separation offers higher precision and efficiency in isotopic enrichment compared to traditional chemical enrichment methods by selectively ionizing specific isotopes using tuned laser frequencies.
Mass spectrometry enrichment
Mass spectrometry-based isotopic enrichment precisely isolates target isotopes by exploiting mass-to-charge differences, offering superior specificity and sensitivity compared to chemical enrichment methods.
Solvent extraction
Solvent extraction offers higher selectivity and efficiency in isotopic enrichment by exploiting subtle differences in isotopic complexes compared to chemical enrichment methods.
Ion exchange chromatography
Ion exchange chromatography enables precise isotopic enrichment by selectively separating isotopes based on their ionic interactions, offering higher specificity compared to broad chemical enrichment methods.
Thermal diffusion
Thermal diffusion achieves isotopic enrichment by exploiting temperature gradients to separate isotopes based on their mass differences, offering a distinct physical method compared to chemical enrichment processes.
Isotopic assay
Isotopic enrichment techniques achieve higher isotopic assay precision by selectively increasing the concentration of specific isotopes, unlike chemical enrichment methods that rely on compound separation without altering isotopic ratios.
Feedstock purity
Isotopic enrichment achieves higher feedstock purity through selective isotope separation, while chemical enrichment relies on chemical reactions that may introduce impurities.
isotopic enrichment vs chemical enrichment Infographic
 njnir.com
njnir.com