Hydrogen fuel cells offer higher energy density and faster refueling times compared to lithium-ion batteries, making them ideal for heavy-duty transport and long-range applications. Lithium-ion batteries excel in energy efficiency and are widely used in portable electronics and electric vehicles due to their established infrastructure and lower upfront costs. Both technologies play complementary roles in advancing sustainable energy systems by reducing greenhouse gas emissions and dependency on fossil fuels.
Table of Comparison
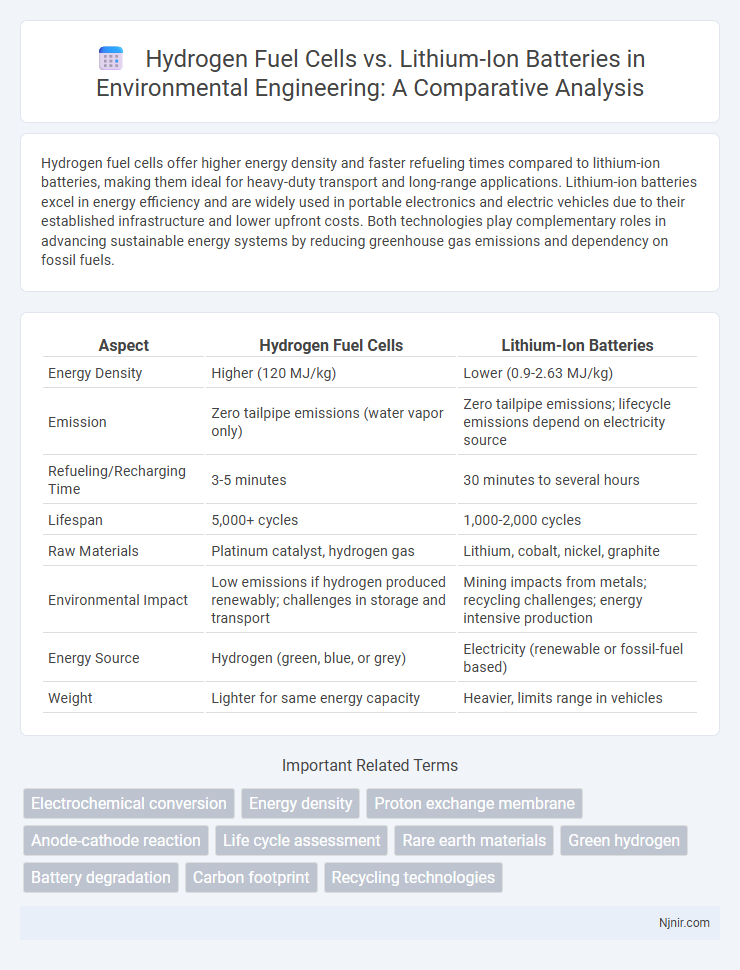
| Aspect | Hydrogen Fuel Cells | Lithium-Ion Batteries |
|---|---|---|
| Energy Density | Higher (120 MJ/kg) | Lower (0.9-2.63 MJ/kg) |
| Emission | Zero tailpipe emissions (water vapor only) | Zero tailpipe emissions; lifecycle emissions depend on electricity source |
| Refueling/Recharging Time | 3-5 minutes | 30 minutes to several hours |
| Lifespan | 5,000+ cycles | 1,000-2,000 cycles |
| Raw Materials | Platinum catalyst, hydrogen gas | Lithium, cobalt, nickel, graphite |
| Environmental Impact | Low emissions if hydrogen produced renewably; challenges in storage and transport | Mining impacts from metals; recycling challenges; energy intensive production |
| Energy Source | Hydrogen (green, blue, or grey) | Electricity (renewable or fossil-fuel based) |
| Weight | Lighter for same energy capacity | Heavier, limits range in vehicles |
Introduction to Hydrogen Fuel Cells and Lithium-Ion Batteries
Hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen, producing only water as a byproduct, making them a clean energy source with high energy density. Lithium-ion batteries store electrical energy chemically and convert it back to electricity, offering high energy efficiency and widespread use in portable electronics and electric vehicles. Both technologies present unique advantages and challenges in energy storage, impacting their adoption in transportation and renewable energy systems.
How Hydrogen Fuel Cells Work
Hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen, producing only water and heat as byproducts, which makes them environmentally friendly. These cells consist of an anode, cathode, and electrolyte membrane where hydrogen molecules split into protons and electrons; the electrons flow through an external circuit to provide power while protons pass through the membrane to combine with oxygen at the cathode. Unlike lithium-ion batteries that store energy chemically, hydrogen fuel cells continuously produce electricity as long as hydrogen fuel is supplied, offering rapid refueling and longer range capabilities for applications such as transportation and stationary power generation.
How Lithium-Ion Batteries Operate
Lithium-ion batteries operate through the movement of lithium ions between the anode and cathode during charge and discharge cycles, enabling efficient energy storage and release. These batteries rely on electrochemical reactions within a liquid or gel electrolyte to facilitate ion transport, providing high energy density and long cycle life for applications like electric vehicles and portable electronics. Their ability to deliver consistent voltage and rapid recharge capabilities distinguishes them from hydrogen fuel cells, which convert chemical energy directly into electricity through hydrogen oxidation.
Environmental Impact: Hydrogen vs. Lithium-Ion
Hydrogen fuel cells produce water vapor as the only emission, offering a zero-carbon energy source when hydrogen is generated from renewable resources. Lithium-ion batteries, while efficient for energy storage, involve mining for metals like lithium, cobalt, and nickel, causing significant environmental degradation and resource depletion. Recycling challenges and battery disposal issues further elevate the environmental impact associated with widespread lithium-ion adoption.
Energy Efficiency Comparison
Hydrogen fuel cells demonstrate an energy efficiency range of 40-60%, converting chemical energy directly into electricity with water as the only byproduct. Lithium-ion batteries typically achieve higher round-trip efficiencies of 85-95%, storing and releasing electrical energy with minimal losses. The disparity in efficiency influences their suitability for different applications, with lithium-ion batteries favored for short-term, high-efficiency energy storage and hydrogen fuel cells suited for long-range energy needs and rapid refueling.
Raw Material Extraction and Sustainability
Hydrogen fuel cells primarily rely on platinum catalysts and require water electrolysis, which involves mining of rare metals and significant energy input, while lithium-ion batteries depend heavily on cobalt, lithium, and nickel extraction, causing environmental degradation and social concerns in mining regions. The sustainability of hydrogen fuel cells improves with green hydrogen production using renewable energy, reducing carbon emissions compared to lithium-ion batteries whose lifecycle impact is tied to resource-intensive mining and recycling challenges. Recycling and resource recovery technologies are advancing for both, but current lithium-ion battery supply chains show greater risks of supply bottlenecks and ecological harm than the emerging green hydrogen infrastructure.
Storage and Transportation Challenges
Hydrogen fuel cells face storage challenges due to hydrogen's low volumetric energy density, requiring high-pressure tanks or cryogenic temperatures, which complicate transportation and infrastructure. Lithium-ion batteries offer higher energy density and simpler transport logistics but suffer from weight limitations and degradation over repeated cycles. Both technologies encounter specific hurdles in energy storage efficiency and safe, cost-effective transportation solutions.
Renewable Energy Integration
Hydrogen fuel cells offer longer energy storage duration and rapid refueling capabilities, making them ideal for balancing intermittent renewable energy sources such as solar and wind. Lithium-ion batteries excel at providing high-efficiency short-term storage with fast response times, supporting grid stability and peak load management from renewable generation. Integrating both technologies creates a hybrid energy system that maximizes renewable energy utilization and enhances grid resilience.
Life Cycle Analysis and Longevity
Hydrogen fuel cells exhibit a favorable life cycle analysis with significantly lower carbon emissions during operation compared to lithium-ion batteries, which involve intensive resource extraction and complex recycling processes. Hydrogen fuel cells typically achieve longer operational longevity, with durability often exceeding 5,000 cycles, whereas lithium-ion batteries commonly degrade after 1,000 to 1,500 charge-discharge cycles. The overall sustainability of hydrogen fuel cells benefits from renewable hydrogen production and modular designs, contrasting with lithium-ion batteries' dependence on rare metals and energy-intensive manufacturing.
Future Prospects in Clean Transportation
Hydrogen fuel cells offer higher energy density and faster refueling times compared to lithium-ion batteries, positioning them as a promising solution for long-haul transportation and heavy-duty vehicles. Lithium-ion batteries dominate current electric vehicles with established charging infrastructure and cost reductions but face limitations in range and charging speed. Future advancements in hydrogen production, fuel cell durability, and battery energy density will determine their respective roles in decarbonizing the transportation sector.
Electrochemical conversion
Hydrogen fuel cells convert chemical energy into electricity through an electrochemical reaction between hydrogen and oxygen, while lithium-ion batteries store and release energy via reversible electrochemical reactions involving lithium ions moving between electrodes.
Energy density
Hydrogen fuel cells offer significantly higher energy density compared to lithium-ion batteries, enabling longer range and lighter power storage for applications like electric vehicles and portable devices.
Proton exchange membrane
Proton exchange membrane hydrogen fuel cells offer higher energy density and faster refueling times compared to lithium-ion batteries, making them more suitable for long-range and heavy-duty applications.
Anode-cathode reaction
Hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen at the anode and oxygen at the cathode, producing water as a byproduct, while lithium-ion batteries rely on lithium ions moving between the anode and cathode during charge and discharge cycles to store and release energy.
Life cycle assessment
Hydrogen fuel cells generally exhibit lower environmental impact in long-term life cycle assessments due to higher energy density and faster refueling, whereas lithium-ion batteries often face challenges related to resource extraction, recyclability, and shorter lifespan.
Rare earth materials
Hydrogen fuel cells rely less on rare earth materials compared to lithium-ion batteries, which require significant amounts of rare earth elements like cobalt and nickel for cathode production.
Green hydrogen
Green hydrogen fuel cells offer a sustainable energy solution with zero emissions and higher energy density compared to lithium-ion batteries, making them ideal for large-scale, long-duration storage and heavy-duty transportation.
Battery degradation
Hydrogen fuel cells exhibit minimal degradation over time compared to lithium-ion batteries, which typically suffer capacity loss and reduced lifespan due to repeated charge-discharge cycles.
Carbon footprint
Hydrogen fuel cells produce significantly lower carbon footprints than lithium-ion batteries, especially when hydrogen is generated from renewable sources, reducing lifecycle greenhouse gas emissions in transportation and energy storage.
Recycling technologies
Hydrogen fuel cells offer easier recycling through simpler materials like platinum and membranes, while lithium-ion battery recycling requires advanced processes to recover valuable metals such as cobalt, nickel, and lithium efficiently.
hydrogen fuel cells vs lithium-ion batteries Infographic
 njnir.com
njnir.com