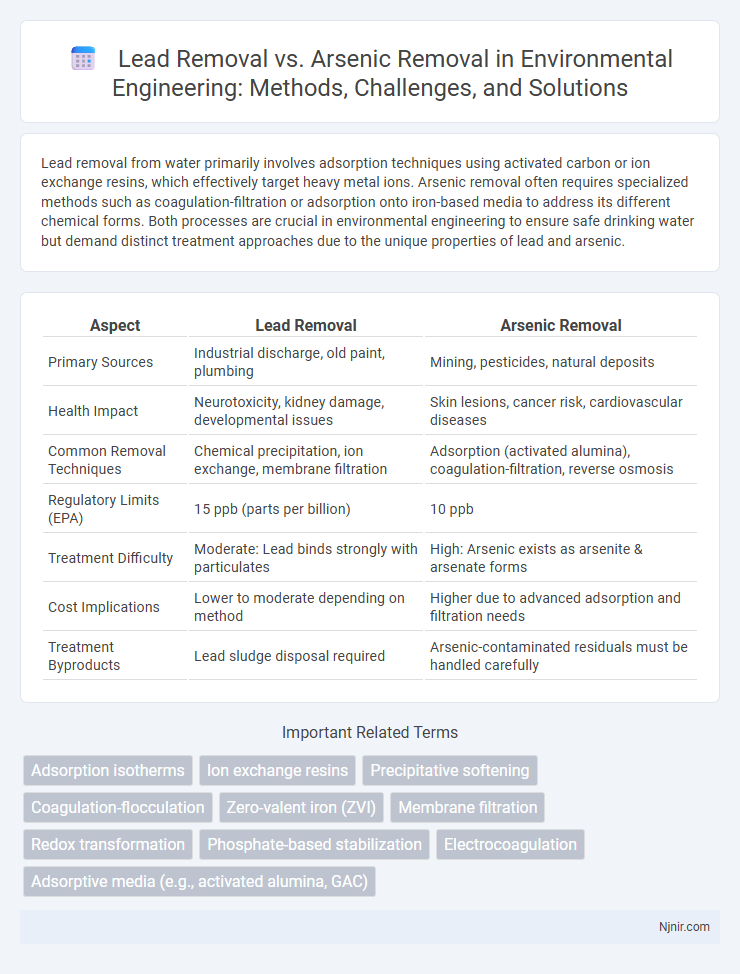
Lead removal from water primarily involves adsorption techniques using activated carbon or ion exchange resins, which effectively target heavy metal ions. Arsenic removal often requires specialized methods such as coagulation-filtration or adsorption onto iron-based media to address its different chemical forms. Both processes are crucial in environmental engineering to ensure safe drinking water but demand distinct treatment approaches due to the unique properties of lead and arsenic.
Table of Comparison
| Aspect | Lead Removal | Arsenic Removal |
|---|---|---|
| Primary Sources | Industrial discharge, old paint, plumbing | Mining, pesticides, natural deposits |
| Health Impact | Neurotoxicity, kidney damage, developmental issues | Skin lesions, cancer risk, cardiovascular diseases |
| Common Removal Techniques | Chemical precipitation, ion exchange, membrane filtration | Adsorption (activated alumina), coagulation-filtration, reverse osmosis |
| Regulatory Limits (EPA) | 15 ppb (parts per billion) | 10 ppb |
| Treatment Difficulty | Moderate: Lead binds strongly with particulates | High: Arsenic exists as arsenite & arsenate forms |
| Cost Implications | Lower to moderate depending on method | Higher due to advanced adsorption and filtration needs |
| Treatment Byproducts | Lead sludge disposal required | Arsenic-contaminated residuals must be handled carefully |
Overview of Lead and Arsenic Contamination
Lead contamination primarily originates from industrial discharge, aging plumbing systems, and lead-based paints, posing severe neurological and developmental health risks, especially in children. Arsenic contamination commonly results from natural geological deposits leaching into groundwater, along with agricultural runoff and mining activities, linked to skin lesions, cancer, and cardiovascular diseases. Both contaminants require specialized removal techniques due to their distinct sources and chemical behaviors in water systems.
Sources of Lead vs Arsenic in Water Systems
Lead contamination in water systems primarily originates from corroded plumbing materials such as old pipes, fixtures, and solder used in household plumbing and water distribution systems. Arsenic presence in water typically comes from natural sources like the erosion of arsenic-rich minerals and industrial activities such as mining and use of arsenic-containing pesticides. Understanding these distinct sources is crucial for selecting appropriate water treatment methods tailored to effectively remove either lead or arsenic from drinking water.
Health Impacts: Lead Exposure vs Arsenic Exposure
Lead exposure primarily causes neurological damage, developmental delays in children, and cardiovascular problems in adults, while arsenic exposure is linked to skin lesions, various cancers, and cardiovascular diseases. Both contaminants pose serious health risks, but lead's neurotoxicity particularly affects cognitive function and behavior, whereas arsenic's carcinogenic properties result in long-term chronic illnesses. Effective removal of lead and arsenic from drinking water is critical to preventing these severe health outcomes and protecting vulnerable populations.
Regulatory Standards for Lead and Arsenic Removal
Regulatory standards for lead and arsenic removal are established by agencies like the EPA under the Safe Drinking Water Act, setting Maximum Contaminant Levels (MCLs) at 0.015 mg/L for lead and 0.010 mg/L for arsenic. Lead removal technologies often emphasize point-of-use filters certified to reduce lead below action levels, while arsenic removal requires specialized treatments such as adsorption media or reverse osmosis to meet stricter MCLs due to arsenic's higher toxicity. Compliance with these regulations ensures public health safety by controlling heavy metal contamination in drinking water systems.
Lead Removal Technologies and Methods
Lead removal technologies primarily include ion exchange, reverse osmosis, and chemical precipitation, each effectively reducing lead concentrations below the EPA's action level of 15 ppb. Ion exchange resins specifically target lead ions, offering high selectivity and efficiency in both residential and industrial water treatment systems. Reverse osmosis membranes provide a physical barrier to lead particles, delivering comprehensive purification while chemical precipitation converts dissolved lead into insoluble compounds for easy filtration.
Arsenic Removal Technologies and Methods
Arsenic removal technologies primarily include adsorption using activated alumina, ion exchange resins, and coagulation-filtration methods to effectively reduce arsenic concentrations in water. Advanced treatments like reverse osmosis and ultrafiltration membranes provide high efficiency by physically separating arsenic particles from water sources. Emerging methods, such as electrocoagulation and biological treatments, offer sustainable and cost-effective alternatives for arsenic remediation in drinking water systems.
Comparative Effectiveness of Removal Techniques
Lead removal commonly employs methods such as ion exchange, activated carbon filtration, and reverse osmosis, each offering varying degrees of effectiveness depending on water chemistry and contaminant concentration. Arsenic removal techniques often include coagulation-filtration, adsorption media containing iron oxides, and membrane filtration, with adsorption showing high specificity for arsenic species. Comparative studies indicate reverse osmosis provides robust removal for both contaminants, while ion exchange is more cost-effective for lead but less efficient for arsenic, emphasizing the need for site-specific water testing to select optimal treatment methods.
Cost and Sustainability Considerations
Lead removal technologies often involve chemical precipitation or ion exchange, which can have moderate operational costs but generate hazardous sludge requiring careful disposal, impacting sustainability. Arsenic removal methods, such as adsorption using activated alumina or iron-based media, typically demand higher initial investment but offer regenerable media options, reducing long-term waste production and environmental footprint. Evaluating cost-effectiveness must balance treatment efficiency, media lifespan, and waste management to ensure sustainable implementation in water purification systems.
Challenges in Implementing Removal Solutions
Implementing lead removal solutions often faces challenges like corrosion control in plumbing systems and the need for point-of-use filters to ensure safety, while arsenic removal requires dealing with its complex chemical forms that vary by location, complicating treatment processes. Both contaminants demand continuous monitoring to maintain regulatory compliance and prevent recontamination, increasing operational costs. Limited access to advanced technologies and the high cost of infrastructure upgrades further hinder widespread adoption of effective removal methods for both lead and arsenic in water supplies.
Future Innovations in Lead and Arsenic Remediation
Future innovations in lead and arsenic remediation emphasize advanced nanotechnology and bio-remediation techniques to enhance contaminant adsorption efficiency and reduce environmental impact. Emerging electrochemical methods and smart sensors offer real-time monitoring and targeted removal, improving precision in water treatment systems. Integration of artificial intelligence and machine learning algorithms optimizes process control and predicts contaminant fluctuations, driving the next generation of sustainable lead and arsenic removal solutions.
Adsorption isotherms
Adsorption isotherms reveal that lead removal typically exhibits higher adsorption capacity on activated carbon compared to arsenic, due to distinct chemical affinities influencing metal ion uptake efficiency.
Ion exchange resins
Ion exchange resins effectively remove both lead and arsenic from water by selectively exchanging harmful ions with benign ones, but resin formulation and operational parameters differ to optimize performance for each contaminant.
Precipitative softening
Precipitative softening effectively removes lead by converting soluble lead salts into insoluble precipitates, but it is less efficient for arsenic removal, which often requires adsorption or ion exchange methods.
Coagulation-flocculation
Coagulation-flocculation effectively removes lead by aggregating metallic particles, while arsenic removal requires specific coagulants like ferric chloride to precipitate arsenic species efficiently.
Zero-valent iron (ZVI)
Zero-valent iron (ZVI) exhibits high efficiency in removing both lead and arsenic from water through reductive precipitation and adsorption, with optimized ZVI particle size and surface modification enhancing arsenic removal kinetics compared to lead.
Membrane filtration
Membrane filtration effectively removes lead and arsenic from water by employing ultrafiltration and reverse osmosis technologies that target heavy metal ions and arsenic compounds with high rejection rates.
Redox transformation
Redox transformation efficiently converts toxic lead and arsenic species into less soluble and less harmful forms, enhancing the effectiveness of removal processes in water treatment.
Phosphate-based stabilization
Phosphate-based stabilization effectively immobilizes lead by forming insoluble lead phosphate minerals, while it is less effective for arsenic removal, which often requires adsorption or coagulation methods for optimal remediation.
Electrocoagulation
Electrocoagulation effectively removes lead and arsenic from water by using an electric current to destabilize and aggregate contaminants, with lead ions typically precipitating faster due to their higher reactivity compared to arsenic species.
Adsorptive media (e.g., activated alumina, GAC)
Adsorptive media such as activated alumina effectively remove arsenic from water by targeting arsenate and arsenite species, while granular activated carbon (GAC) excels in lead removal through strong adsorption of lead ions and complexed lead compounds.
lead removal vs arsenic removal Infographic
 njnir.com
njnir.com