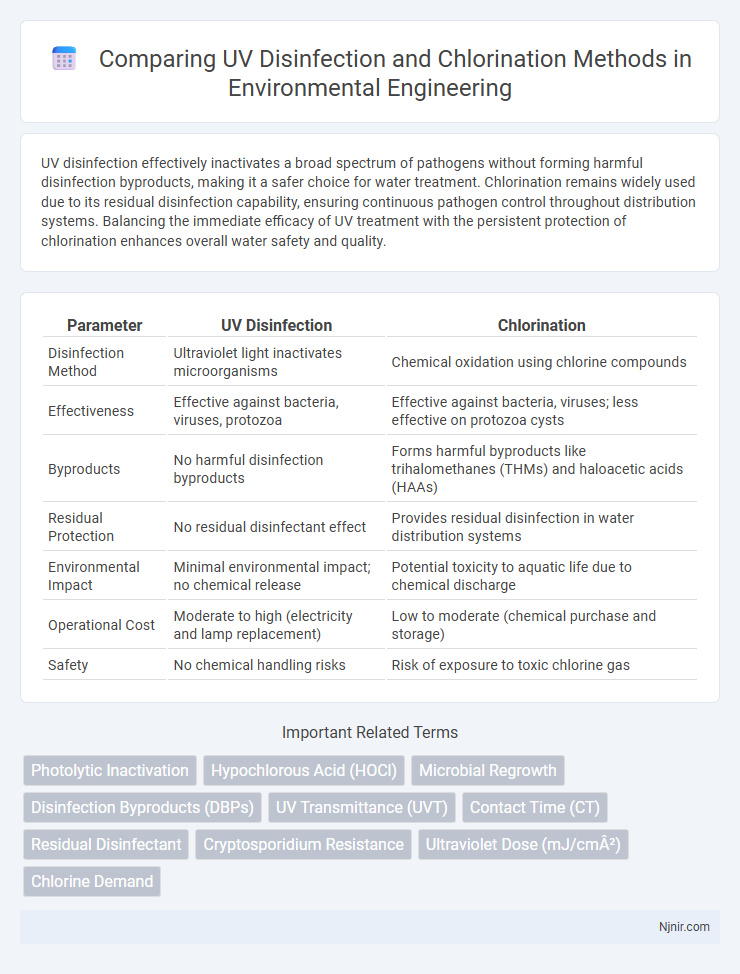
UV disinfection effectively inactivates a broad spectrum of pathogens without forming harmful disinfection byproducts, making it a safer choice for water treatment. Chlorination remains widely used due to its residual disinfection capability, ensuring continuous pathogen control throughout distribution systems. Balancing the immediate efficacy of UV treatment with the persistent protection of chlorination enhances overall water safety and quality.
Table of Comparison
| Parameter | UV Disinfection | Chlorination |
|---|---|---|
| Disinfection Method | Ultraviolet light inactivates microorganisms | Chemical oxidation using chlorine compounds |
| Effectiveness | Effective against bacteria, viruses, protozoa | Effective against bacteria, viruses; less effective on protozoa cysts |
| Byproducts | No harmful disinfection byproducts | Forms harmful byproducts like trihalomethanes (THMs) and haloacetic acids (HAAs) |
| Residual Protection | No residual disinfectant effect | Provides residual disinfection in water distribution systems |
| Environmental Impact | Minimal environmental impact; no chemical release | Potential toxicity to aquatic life due to chemical discharge |
| Operational Cost | Moderate to high (electricity and lamp replacement) | Low to moderate (chemical purchase and storage) |
| Safety | No chemical handling risks | Risk of exposure to toxic chlorine gas |
Introduction to Water Disinfection Methods
UV disinfection and chlorination are two primary water disinfection methods used to eliminate pathogens and ensure safe drinking water. UV disinfection uses ultraviolet light to inactivate bacteria, viruses, and protozoa by damaging their DNA, while chlorination involves adding chlorine chemicals to destroy microorganisms through oxidation. Both methods are effective but differ in operational costs, chemical usage, residual disinfection capability, and treatment byproducts.
Overview of UV Disinfection Technology
UV disinfection technology uses ultraviolet light at a germicidal wavelength of 254 nanometers to inactivate microorganisms by damaging their DNA and RNA, preventing reproduction. This method offers chemical-free water treatment, eliminating pathogens such as bacteria, viruses, and protozoa without forming harmful disinfection byproducts like trihalomethanes commonly associated with chlorination. UV systems are energy-efficient, require minimal maintenance, and provide instantaneous disinfection, making them ideal for municipal, industrial, and residential water purification.
Fundamentals of Chlorination Process
Chlorination involves adding chlorine or chlorine compounds to water, releasing hypochlorous acid that effectively kills bacteria, viruses, and other pathogens through oxidation and disruption of cellular processes. This process depends on factors such as chlorine concentration, contact time, pH level, and temperature to achieve optimal disinfection efficacy. Chlorination also provides a residual disinfectant effect, maintaining water safety throughout distribution systems, unlike UV disinfection which lacks residual protection.
Efficacy Against Waterborne Pathogens
UV disinfection effectively inactivates a broad spectrum of waterborne pathogens, including bacteria, viruses, and protozoa such as Cryptosporidium and Giardia, by damaging their DNA or RNA, which prevents replication. Chlorination provides residual disinfection and is highly effective against most bacteria and viruses but may have limited efficacy against chlorine-resistant protozoa and can form harmful disinfection byproducts (DBPs). UV systems offer rapid pathogen inactivation without chemical residues, while chlorination ensures ongoing protection in distribution systems through a lasting disinfectant residual.
Environmental Impact of UV vs Chlorination
UV disinfection reduces environmental impact by eliminating harmful disinfection byproducts common in chlorination, such as trihalomethanes and haloacetic acids, which can contaminate water and soil. Unlike chlorination, UV treatment does not introduce toxic residual chemicals into the environment, minimizing risks to aquatic ecosystems and reducing the need for complex chemical handling and storage. Energy consumption for UV systems is generally lower, and waste generation is minimal, making UV disinfection a more sustainable and eco-friendly water treatment option.
Chemical Byproducts and Residuals
UV disinfection effectively inactivates pathogens without generating harmful chemical byproducts, ensuring no residual chemicals remain in treated water. Chlorination, while providing a persistent disinfectant residual, can produce disinfection byproducts (DBPs) such as trihalomethanes (THMs) and haloacetic acids (HAAs) linked to health risks. The absence of residual disinfectants in UV treatment requires precise system design to maintain microbial safety throughout distribution systems.
Operational and Maintenance Requirements
UV disinfection systems require regular cleaning of lamps and sleeves to maintain effective UV intensity and periodic lamp replacement, typically every 9,000 to 12,000 hours, while chlorination demands continuous chemical monitoring and frequent replenishment of chlorine supplies. UV systems necessitate electrical power and routine inspection of electronic components, whereas chlorination involves managing chemical storage safety and handling hazards. Both methods require trained personnel for operation, but chlorination often entails more complex safety protocols due to the toxic nature of chlorine gas or liquid.
Cost Analysis: UV Disinfection vs Chlorination
UV disinfection systems typically incur higher upfront capital costs compared to chlorination, but offer lower long-term operational expenses due to reduced chemical usage and maintenance requirements. Chlorination has lower initial investment costs but involves continuous spending on chemicals, storage, and safety measures, which can increase total lifecycle costs. Considering energy consumption, UV systems may have higher electricity costs, while chlorination involves risks of chemical handling that could lead to additional regulatory and environmental expenses.
Regulatory Compliance and Safety Considerations
UV disinfection complies with stringent EPA and WHO regulations by effectively eliminating pathogens without producing harmful disinfection byproducts, ensuring safer water treatment. Chlorination must meet EPA standards but often generates potentially carcinogenic byproducts like trihalomethanes, raising safety concerns under regulatory oversight. Facilities prioritizing regulatory compliance and safety increasingly adopt UV systems due to their non-toxic, residue-free nature and minimal environmental impact.
Future Trends in Disinfection Technologies
UV disinfection is gaining momentum due to its chemical-free process, reducing harmful byproducts compared to traditional chlorination methods. Emerging technologies integrate advanced UV-LEDs for energy efficiency and enhanced microbial inactivation, signaling a shift toward sustainable water treatment solutions. Smart sensor systems and IoT are increasingly incorporated, allowing real-time monitoring and optimization of disinfection efficacy in future water treatment infrastructures.
Photolytic Inactivation
UV disinfection achieves photolytic inactivation by damaging microbial DNA and RNA through UV-C light exposure, offering a chemical-free alternative to chlorination that reduces harmful byproducts and enhances water safety.
Hypochlorous Acid (HOCl)
Hypochlorous acid (HOCl) in chlorination acts as a powerful disinfectant by penetrating microbial cell walls, while UV disinfection inactivates pathogens through DNA damage without chemical residues.
Microbial Regrowth
UV disinfection effectively inhibits microbial regrowth by damaging DNA in pathogens, whereas chlorination can allow microbial rebound due to residual chlorine depletion over time.
Disinfection Byproducts (DBPs)
UV disinfection eliminates harmful disinfection byproducts (DBPs) commonly produced during chlorination, ensuring safer water treatment without chemical residues.
UV Transmittance (UVT)
UV disinfection efficiency critically depends on high UV Transmittance (UVT) levels in water, whereas chlorination effectiveness is less influenced by UVT but more affected by organic matter presence.
Contact Time (CT)
UV disinfection achieves effective pathogen inactivation with significantly shorter contact time (CT) compared to chlorination, which requires longer CT to ensure complete microbial destruction.
Residual Disinfectant
UV disinfection provides no residual disinfectant in the water, whereas chlorination maintains a residual level that continues to protect against microbial contamination throughout the distribution system.
Cryptosporidium Resistance
UV disinfection effectively inactivates Cryptosporidium oocysts resistant to chlorination, providing a more reliable solution for controlling this waterborne parasite.
Ultraviolet Dose (mJ/cm²)
Ultraviolet disinfection requires a UV dose typically ranging from 30 to 100 mJ/cm2 for effective pathogen inactivation, whereas chlorination effectiveness depends on chlorine concentration and contact time rather than UV dose.
Chlorine Demand
Chlorine demand in water treatment significantly increases operational costs and complexity compared to UV disinfection, which requires no chemical additives or residual monitoring.
UV Disinfection vs Chlorination Infographic
 njnir.com
njnir.com