Ozone treatment effectively disinfects water by breaking down contaminants through oxidation but can be limited by the formation of harmful by-products. Advanced oxidation processes (AOPs) generate highly reactive hydroxyl radicals that enhance the degradation of persistent organic pollutants more efficiently than ozone alone. Combining ozone with AOPs improves overall treatment performance, ensuring greater removal of complex contaminants in environmental engineering applications.
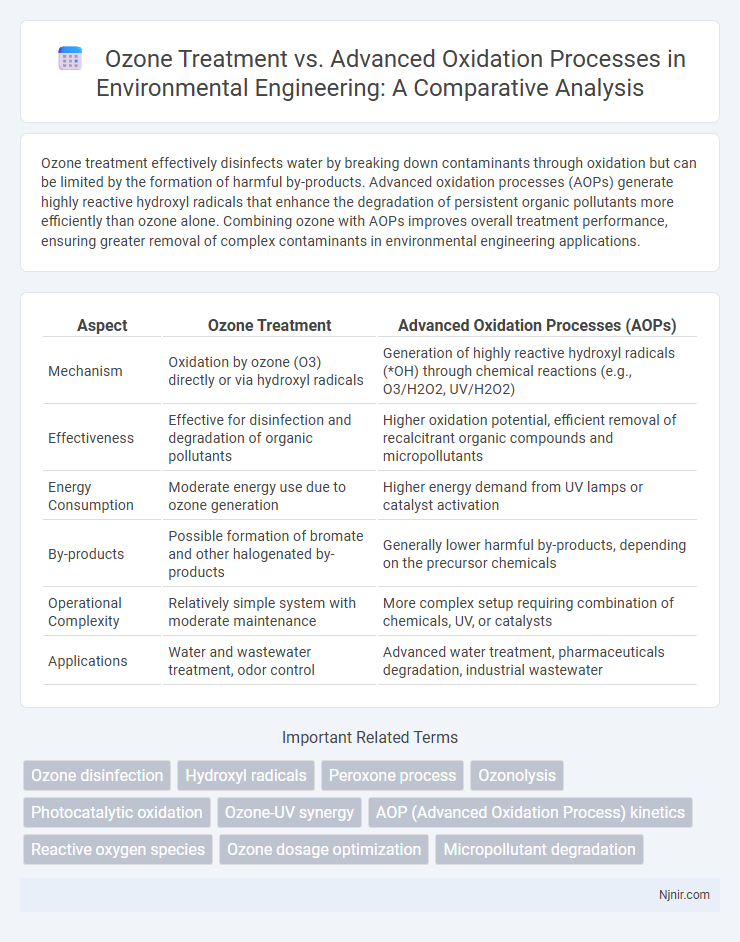
Table of Comparison
| Aspect | Ozone Treatment | Advanced Oxidation Processes (AOPs) |
|---|---|---|
| Mechanism | Oxidation by ozone (O3) directly or via hydroxyl radicals | Generation of highly reactive hydroxyl radicals (*OH) through chemical reactions (e.g., O3/H2O2, UV/H2O2) |
| Effectiveness | Effective for disinfection and degradation of organic pollutants | Higher oxidation potential, efficient removal of recalcitrant organic compounds and micropollutants |
| Energy Consumption | Moderate energy use due to ozone generation | Higher energy demand from UV lamps or catalyst activation |
| By-products | Possible formation of bromate and other halogenated by-products | Generally lower harmful by-products, depending on the precursor chemicals |
| Operational Complexity | Relatively simple system with moderate maintenance | More complex setup requiring combination of chemicals, UV, or catalysts |
| Applications | Water and wastewater treatment, odor control | Advanced water treatment, pharmaceuticals degradation, industrial wastewater |
Introduction to Ozone Treatment and Advanced Oxidation Processes
Ozone treatment utilizes ozone (O3), a powerful oxidant, to effectively degrade organic pollutants and disinfect water by breaking down contaminants through direct oxidation or by forming hydroxyl radicals. Advanced oxidation processes (AOPs) combine ozone with other oxidants such as hydrogen peroxide or UV light, enhancing the generation of hydroxyl radicals to achieve more efficient and rapid degradation of complex contaminants. Both ozone treatment and AOPs are critical technologies in wastewater treatment, offering high reactivity, broad-spectrum contaminant removal, and minimal harmful by-product formation.
Mechanisms of Pollutant Removal: Ozone vs. AOPs
Ozone treatment removes pollutants primarily through direct oxidation, where ozone molecules react with organic and inorganic contaminants, breaking them down into less harmful substances. Advanced Oxidation Processes (AOPs) generate highly reactive hydroxyl radicals (*OH) that non-selectively degrade a broad range of pollutants with higher efficiency and faster reaction rates than ozone alone. The superior oxidizing potential of AOPs results in more complete mineralization of complex organic compounds, reducing residual toxicity in treated water.
Key Applications in Environmental Engineering
Ozone treatment is highly effective in disinfecting water and air by oxidizing organic pollutants and pathogens in wastewater treatment plants and industrial effluent management. Advanced oxidation processes (AOPs) combine ozone with hydrogen peroxide, UV light, or catalysts to generate hydroxyl radicals that degrade recalcitrant organic contaminants in soil remediation and advanced water purification systems. Both technologies are critical in environmental engineering for improving water quality, controlling odor, and enhancing the breakdown of toxic substances in municipal and industrial environments.
Efficiency in Treating Organic and Inorganic Contaminants
Ozone treatment demonstrates high efficiency in degrading a wide range of organic contaminants due to its strong oxidative potential and ability to break down complex molecules into simpler compounds. Advanced oxidation processes (AOPs), which combine ozone with other oxidants or catalysts like hydrogen peroxide or UV light, significantly enhance the removal of both organic and inorganic pollutants by generating highly reactive hydroxyl radicals. These hydroxyl radicals provide superior oxidation power, enabling AOPs to achieve faster reaction rates and more complete mineralization compared to ozone treatment alone.
Operational Parameters and Optimization
Ozone treatment relies heavily on parameters such as ozone dose, contact time, and pH to maximize pollutant degradation and disinfection efficiency. Advanced oxidation processes (AOPs) optimize performance by controlling factors like oxidant concentration, UV radiation intensity, and catalyst presence, which enhance hydroxyl radical generation for faster and more complete contaminant breakdown. Both methods require careful adjustment of operational parameters to balance treatment efficacy, energy consumption, and byproduct formation for sustainable water and air purification.
Comparative Analysis of By-product Formation
Ozone treatment generates by-products such as bromate and aldehydes, which pose health risks requiring strict regulatory limits. Advanced Oxidation Processes (AOPs) produce hydroxyl radicals that degrade contaminants more completely, resulting in fewer and less harmful by-products like trace organic acids. Comparative analysis shows that AOPs generally offer superior control over toxic by-product formation, enhancing water treatment safety and environmental compliance.
Energy Consumption and Cost Considerations
Ozone treatment generally requires lower energy consumption compared to advanced oxidation processes (AOPs), as ozonation relies mainly on ozone generation which is energy-efficient. In contrast, AOPs often involve combining ozone with hydrogen peroxide or UV light, significantly increasing operational costs due to higher energy demands and chemical inputs. Cost considerations favor ozone treatment for large-scale water treatment facilities aiming to balance efficiency with reduced energy expenditure.
Environmental Impact and Sustainability
Ozone treatment generates fewer harmful byproducts and degrades pollutants efficiently, making it a greener option in water and air purification compared to traditional chemical methods. Advanced Oxidation Processes (AOPs) employ hydroxyl radicals with high oxidation potential, but often require significant energy input and complex catalysts, potentially increasing environmental footprint. Evaluating lifecycle emissions and resource use favors ozone treatment for sustainability, though integrating AOPs with renewable energy sources can mitigate environmental impact in advanced contaminant removal.
Challenges and Limitations of Each Method
Ozone treatment faces challenges such as limited efficacy against certain contaminants, high operational costs, and the potential formation of harmful byproducts like bromate. Advanced oxidation processes (AOPs) exhibit limitations including complex system design, high energy consumption, and the need for precise control of reaction conditions to avoid incomplete pollutant degradation. Both technologies require careful optimization to balance treatment efficiency with operational feasibility and environmental safety.
Future Trends in Oxidation Technologies for Water and Air Treatment
Future trends in oxidation technologies for water and air treatment emphasize the integration of ozone treatment with advanced oxidation processes (AOPs) to enhance pollutant degradation efficiency and reduce by-product formation. Innovations focus on hybrid systems combining ozone with ultraviolet (UV) radiation, hydrogen peroxide, or catalysts like titanium dioxide to generate highly reactive hydroxyl radicals that accelerate contaminant breakdown. Scaling up these hybrid ozone-AOP systems, supported by real-time monitoring and AI-driven process optimization, is projected to improve sustainability and cost-effectiveness in large-scale water and air purification applications.
Ozone disinfection
Ozone disinfection effectively eliminates bacteria, viruses, and organic contaminants by generating powerful oxidative radicals that disrupt microbial cell walls and degrade pollutants faster than many advanced oxidation processes.
Hydroxyl radicals
Hydroxyl radicals generated in advanced oxidation processes exhibit higher reactivity and broader contaminant degradation efficiency than ozone treatment alone in water purification.
Peroxone process
The Peroxone process, combining ozone and hydrogen peroxide, significantly enhances advanced oxidation by generating hydroxyl radicals that improve pollutant degradation efficiency compared to ozone treatment alone.
Ozonolysis
Ozonolysis, a key mechanism in ozone treatment, effectively breaks down complex organic pollutants through targeted oxidation, distinguishing it from broader advanced oxidation processes that rely on multiple reactive species.
Photocatalytic oxidation
Photocatalytic oxidation, a subset of advanced oxidation processes, leverages semiconductor catalysts activated by light to generate reactive oxygen species that efficiently degrade pollutants, offering higher efficacy and selectivity compared to conventional ozone treatment alone.
Ozone-UV synergy
Ozone-UV synergy enhances advanced oxidation processes by generating highly reactive hydroxyl radicals that improve the degradation of organic pollutants in water treatment.
AOP (Advanced Oxidation Process) kinetics
Advanced Oxidation Processes (AOPs) exhibit faster degradation kinetics than ozone treatment by generating highly reactive hydroxyl radicals that enhance contaminant breakdown rates.
Reactive oxygen species
Ozone treatment generates reactive oxygen species such as hydroxyl radicals and singlet oxygen, while advanced oxidation processes produce higher concentrations of hydroxyl radicals, enhancing pollutant degradation efficiency.
Ozone dosage optimization
Optimizing ozone dosage in ozone treatment enhances pollutant degradation efficiency while minimizing operational costs compared to conventional advanced oxidation processes.
Micropollutant degradation
Ozone treatment effectively degrades micropollutants through selective oxidation, while advanced oxidation processes generate highly reactive hydroxyl radicals that achieve faster and more complete mineralization of diverse micropollutants in water treatment.
ozone treatment vs advanced oxidation processes Infographic
 njnir.com
njnir.com