Microplastic removal in environmental engineering primarily involves physical filtration and advanced oxidation processes to capture and degrade tiny plastic particles from water sources. Heavy metal removal, on the other hand, relies on chemical precipitation, ion exchange, and adsorption techniques to extract toxic metal ions from contaminated environments. Both removal methods are crucial for protecting aquatic ecosystems but require tailored approaches due to the differing chemical and physical properties of microplastics and heavy metals.
Table of Comparison
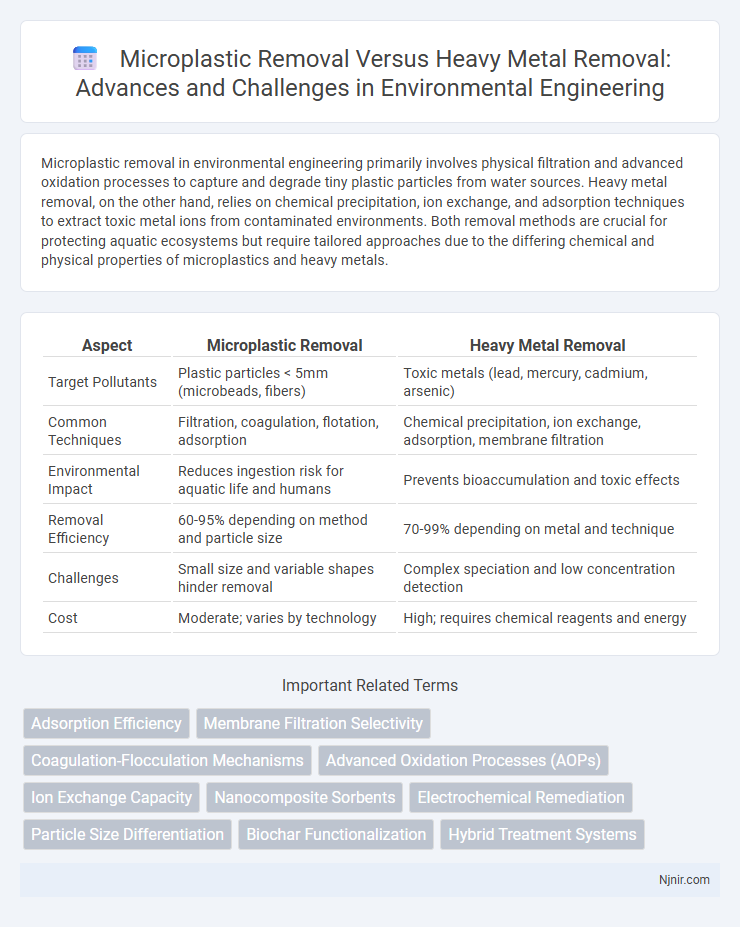
| Aspect | Microplastic Removal | Heavy Metal Removal |
|---|---|---|
| Target Pollutants | Plastic particles < 5mm (microbeads, fibers) | Toxic metals (lead, mercury, cadmium, arsenic) |
| Common Techniques | Filtration, coagulation, flotation, adsorption | Chemical precipitation, ion exchange, adsorption, membrane filtration |
| Environmental Impact | Reduces ingestion risk for aquatic life and humans | Prevents bioaccumulation and toxic effects |
| Removal Efficiency | 60-95% depending on method and particle size | 70-99% depending on metal and technique |
| Challenges | Small size and variable shapes hinder removal | Complex speciation and low concentration detection |
| Cost | Moderate; varies by technology | High; requires chemical reagents and energy |
Introduction to Microplastic and Heavy Metal Pollution
Microplastic pollution consists of tiny plastic particles under 5 millimeters, originating from the breakdown of larger plastics and daily human activities, contaminating oceans, freshwater, and soil globally. Heavy metal pollution involves toxic elements such as lead, mercury, cadmium, and arsenic, released through industrial processes, mining, and improper waste disposal, posing serious risks to ecosystems and human health. Both pollutants persist in the environment, disrupting biological functions and entering food chains, necessitating advanced removal technologies for effective remediation.
Sources and Environmental Impact of Microplastics
Microplastics primarily originate from the breakdown of larger plastic debris, synthetic textiles, and personal care products, posing widespread environmental hazards by contaminating marine ecosystems and entering the food chain. Their small size and persistence lead to bioaccumulation in aquatic organisms, causing harmful effects on biodiversity and human health. In contrast, heavy metals such as lead, mercury, and cadmium stem from industrial discharge, mining, and agricultural runoff, accumulating in sediments and water, but microplastic pollution presents unique challenges due to its pervasive distribution and complex interactions with organic pollutants.
Sources and Environmental Impact of Heavy Metals
Heavy metals such as lead, mercury, cadmium, and arsenic primarily originate from industrial discharges, mining activities, and improper waste disposal, leading to persistent environmental contamination. These metals accumulate in aquatic ecosystems, posing severe risks to biodiversity through bioaccumulation and biomagnification in the food chain. In contrast to microplastics, heavy metals do not degrade, resulting in long-term toxicity that affects soil quality, water resources, and human health.
Comparative Challenges in Pollutant Detection
Microplastic removal poses challenges in pollutant detection due to the small size, diverse chemical compositions, and complex aggregation behaviors of microplastics in aquatic environments. Heavy metal removal requires highly sensitive detection methods to identify trace concentrations and differentiate between various metal species with different toxicities. Both contaminants demand advanced analytical techniques like spectroscopy and chromatography, but microplastics often necessitate imaging-based solutions while heavy metals rely on electrochemical sensors for effective monitoring.
Physical Removal Techniques: Filtration and Adsorption
Physical removal techniques such as filtration and adsorption play crucial roles in addressing microplastic and heavy metal contamination in water treatment processes. Filtration effectively captures microplastic particles by size exclusion, using membrane or sand filters to trap polymers with diameters ranging from micrometers to millimeters. Adsorption utilizes porous materials like activated carbon or biochar to bind heavy metals through surface interactions, enabling efficient removal of metal ions including lead, cadmium, and mercury from aqueous solutions.
Chemical and Biological Remediation Approaches
Chemical remediation of microplastics involves advanced oxidation processes and surfactant-based coagulation, efficiently breaking down polymer chains or aggregating particles for removal from water. In contrast, heavy metal removal relies on chemical precipitation, ion exchange, and chelation techniques to transform dissolved metal ions into less toxic or insoluble forms. Biological remediation employs specialized microorganisms or plants to metabolize or accumulate microplastics and heavy metals, with bioremediation offering eco-friendly and cost-effective solutions tailored to each contaminant's unique chemical properties.
Efficiency and Limitations of Current Technologies
Microplastic removal technologies, such as membrane filtration and advanced oxidation processes, achieve high efficiency in capturing particles larger than 1 micron but struggle with nanoplastics due to size limitations. Heavy metal removal methods like ion exchange, chemical precipitation, and adsorption offer robust elimination of dissolved metals, reaching removal efficiencies exceeding 90%, yet face challenges with varying metal concentrations and complex water matrices. Current microplastic and heavy metal removal systems both require optimization to address issues like operational costs, scalability, and byproduct management in diverse environmental conditions.
Emerging Innovations in Contaminant Removal
Emerging innovations in contaminant removal highlight advanced filtration membranes and bioengineered bacteria for microplastic removal, enhancing efficiency at micro and nanoscale levels. Heavy metal removal technologies increasingly utilize nanomaterials and electrochemical adsorption methods to target specific metal ions like lead, mercury, and cadmium with high selectivity and minimal energy consumption. Integrating these innovations accelerates water purification processes, addressing complex environmental pollution challenges with sustainable, scalable solutions.
Risk Assessment and Regulatory Standards
Risk assessment for microplastic removal emphasizes the pervasive environmental impact and challenges in detecting nanoplastics, while heavy metal removal focuses on toxicity thresholds and bioaccumulation risks in aquatic ecosystems. Regulatory standards for heavy metals are well-established, with stringent limits set by agencies like the EPA and WHO, whereas microplastic regulations are still emerging, lacking comprehensive guidelines for permissible levels. Effective management requires integrating advanced monitoring techniques and harmonizing global policies to address both contaminants' unique and overlapping health hazards.
Future Perspectives in Integrated Wastewater Treatment
Future perspectives in integrated wastewater treatment highlight advanced technologies combining microplastic removal and heavy metal remediation to enhance environmental safety. Innovations such as biochar adsorption, membrane bioreactors, and nanomaterial-enhanced filtration show potential for simultaneous elimination of microplastics and heavy metals, improving overall treatment efficiency. Research emphasizes developing sustainable, cost-effective systems adaptable for diverse wastewater sources to address rising pollution challenges.
Adsorption Efficiency
Adsorption efficiency for microplastic removal typically varies between 70-95% depending on adsorbent type, while heavy metal removal efficiency can exceed 98% using specialized adsorbents like activated carbon or biochar.
Membrane Filtration Selectivity
Membrane filtration offers higher selectivity for heavy metal removal due to tailored pore sizes and charge interactions, whereas microplastic removal requires optimized membrane structures to capture diverse particle sizes effectively.
Coagulation-Flocculation Mechanisms
Coagulation-flocculation mechanisms efficiently remove microplastics by aggregating polymer particles, whereas heavy metal removal relies on chemical precipitation and adsorption processes to extract metal ions from water.
Advanced Oxidation Processes (AOPs)
Advanced Oxidation Processes (AOPs) effectively degrade microplastics through oxidative fragmentation while simultaneously transforming heavy metals into less toxic, precipitable forms, highlighting their dual capacity in contaminant remediation.
Ion Exchange Capacity
Ion exchange capacity is a critical parameter that determines the efficiency of ion exchange resins in removing heavy metals, while microplastic removal primarily depends on physical filtration and adsorption mechanisms rather than ion exchange capacity.
Nanocomposite Sorbents
Nanocomposite sorbents exhibit superior efficiency in microplastic removal due to their high surface area and selective adsorption properties, while also enabling effective heavy metal removal through tailored functional groups and synergistic interactions.
Electrochemical Remediation
Electrochemical remediation effectively removes heavy metals by converting ions into stable compounds, while microplastic removal requires advanced filtration or adsorption techniques beyond standard electrochemical methods.
Particle Size Differentiation
Microplastic removal targets particles typically larger than 1 micrometer, whereas heavy metal removal focuses on dissolved ions or nanoparticles often below 1 nanometer, requiring distinct filtration and treatment technologies based on particle size differentiation.
Biochar Functionalization
Biochar functionalization enhances selective adsorption capacity, improving microplastic removal through increased surface area and tailored functional groups, while heavy metal removal benefits from ion exchange sites and chelation mechanisms for effective contaminant binding.
Hybrid Treatment Systems
Hybrid treatment systems combining advanced filtration and adsorption techniques enhance the simultaneous removal of microplastics and heavy metals from wastewater, improving overall environmental remediation efficiency.
Microplastic Removal vs Heavy Metal Removal Infographic
 njnir.com
njnir.com