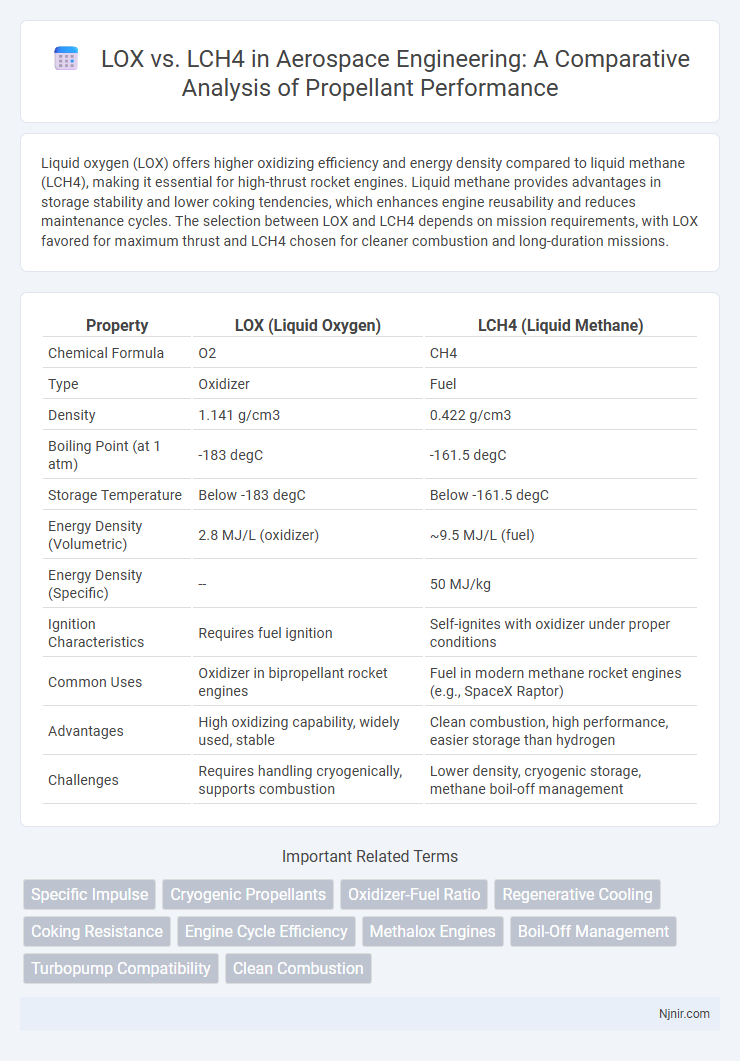
Liquid oxygen (LOX) offers higher oxidizing efficiency and energy density compared to liquid methane (LCH4), making it essential for high-thrust rocket engines. Liquid methane provides advantages in storage stability and lower coking tendencies, which enhances engine reusability and reduces maintenance cycles. The selection between LOX and LCH4 depends on mission requirements, with LOX favored for maximum thrust and LCH4 chosen for cleaner combustion and long-duration missions.
Table of Comparison
| Property | LOX (Liquid Oxygen) | LCH4 (Liquid Methane) |
|---|---|---|
| Chemical Formula | O2 | CH4 |
| Type | Oxidizer | Fuel |
| Density | 1.141 g/cm3 | 0.422 g/cm3 |
| Boiling Point (at 1 atm) | -183 degC | -161.5 degC |
| Storage Temperature | Below -183 degC | Below -161.5 degC |
| Energy Density (Volumetric) | 2.8 MJ/L (oxidizer) | ~9.5 MJ/L (fuel) |
| Energy Density (Specific) | -- | 50 MJ/kg |
| Ignition Characteristics | Requires fuel ignition | Self-ignites with oxidizer under proper conditions |
| Common Uses | Oxidizer in bipropellant rocket engines | Fuel in modern methane rocket engines (e.g., SpaceX Raptor) |
| Advantages | High oxidizing capability, widely used, stable | Clean combustion, high performance, easier storage than hydrogen |
| Challenges | Requires handling cryogenically, supports combustion | Lower density, cryogenic storage, methane boil-off management |
Introduction to LOX and LCH4 Propellants
LOX (Liquid Oxygen) and LCH4 (Liquid Methane) are critical components in modern rocket propulsion, where LOX acts as an oxidizer and LCH4 serves as a fuel. LOX provides high-density oxygen necessary for combustion, enabling efficient energy release when combined with LCH4, which offers clean-burning properties and high specific impulse. This combination is favored in contemporary launch vehicles for its performance advantages and reduced environmental impact compared to traditional propellants.
Chemical Properties and Reaction Mechanisms
Liquid Oxygen (LOX) is a powerful oxidizer with a boiling point of -183degC, characterized by its high reactivity and ability to support rapid combustion through the generation of reactive oxygen species such as singlet oxygen and free radicals. Liquid Methane (LCH4) serves as a hydrocarbon fuel with a boiling point of -161.5degC, undergoing oxidation through a chain-branching mechanism that produces carbon dioxide and water vapor, releasing substantial energy. The reaction mechanism between LOX and LCH4 involves exothermic combustion, where the oxygen molecules dissociate and react with methane's C-H bonds, creating an intense flame with high specific impulse suitable for rocket propulsion.
Energy Performance: Specific Impulse Comparison
LOX (liquid oxygen) combined with LCH4 (liquid methane) exhibits a higher specific impulse (Isp) compared to many other bipropellant combinations, typically reaching values around 350-370 seconds in vacuum conditions. This propellant pair benefits from methane's higher density and cleaner combustion, providing improved energy performance and engine longevity. Specific impulse, a critical metric for propulsion efficiency, highlights LOX/LCH4's advantage in balancing thrust with fuel weight, making it a preferred choice for reusable launch vehicles.
Storage and Handling Challenges
LOX (Liquid Oxygen) requires cryogenic storage at around -183degC, demanding specialized insulated tanks to prevent rapid boil-off and minimize oxygen loss, while stringent safety protocols address its high oxidizing potential and risk of combustion. LCH4 (Liquid Methane) is stored at a warmer cryogenic temperature near -161degC, posing challenges related to methane's flammability, potential leaks, and maintaining structural integrity under low temperatures, necessitating robust containment systems and leak detection methods. Both propellants require meticulous handling to ensure safety, with LOX's aggressive oxidizing nature demanding contamination-free environments and LCH4's volatility requiring rigorous pressure management and ventilation controls.
Safety Considerations
LOX (liquid oxygen) poses significant safety risks due to its extreme oxidizing properties, which can cause rapid combustion and explosions upon contact with flammable materials. LCH4 (liquid methane) offers a lower risk of explosion compared to LOX but presents toxicity and cryogenic hazards, including potential asphyxiation in confined spaces. Proper storage, handling protocols, and leak detection systems are critical to mitigate risks associated with both LOX and LCH4 in aerospace and industrial applications.
Environmental Impact Assessment
Liquid Oxygen (LOX) and Liquid Methane (LCH4) differ significantly in environmental impact when evaluated through Life Cycle Assessment (LCA) metrics. LOX combustion produces only water vapor, resulting in negligible greenhouse gas emissions, while LCH4 combustion emits CO2 and unburned methane, contributing to global warming and atmospheric pollution. Sustainable propulsion systems prioritize LOX for its minimal environmental footprint, whereas LCH4 requires mitigation strategies to reduce methane slip and carbon emissions.
Material Compatibility and Corrosion
LOX (liquid oxygen) presents significant material compatibility challenges due to its strong oxidizing properties, which can induce rapid corrosion and ignition risks in metals like aluminum, steel, and certain alloys not specifically treated or passivated. LCH4 (liquid methane) exhibits lower corrosive potential but still requires careful selection of materials resistant to embrittlement and low-temperature fracture, with stainless steel, certain nickel alloys, and compatible polymers commonly used. Both cryogenic liquids demand rigorous testing for weld integrity, surface finish, and contamination control to ensure longevity and safety in aerospace and industrial applications.
Engine Design Implications
LOX (liquid oxygen) and LCH4 (liquid methane) influence engine design due to their distinct thermophysical properties; LOX offers higher density and oxidizing capacity, enabling compact engine oxidizer turbopumps, while LCH4's higher energy content and cleaner combustion reduce coking and thermal stresses on engine components. The differing stoichiometric mixture ratios and cryogenic temperatures require tailored injector and cooling channel designs to optimize combustion stability and heat transfer efficiency for each propellant combination. Material selection and turbomachinery architecture must accommodate LOX's aggressive oxidation and methane's wider temperature range to balance performance, reliability, and reusability in rocket engine development.
Cost and Accessibility
LOX (liquid oxygen) offers cost advantages due to its widespread industrial production and established supply chains, making it more accessible for various aerospace and industrial applications. LCH4 (liquid methane) tends to have higher production costs and limited infrastructure, resulting in lower accessibility despite its potential as a clean fuel. Cost efficiency and availability of LOX contribute to its preference in many propulsion systems, while LCH4 remains a more specialized choice where performance benefits justify its expense.
Future Trends in Propellant Selection
Future trends in propellant selection favor LOX (liquid oxygen) and LCH4 (liquid methane) due to their efficiency and environmental advantages. LCH4 offers higher specific impulse and cleaner combustion, making it ideal for reusable rockets and long-duration missions on Mars. Advances in storage and handling technologies are expected to improve the feasibility of methane, while LOX remains a staple for its established infrastructure and oxidizing properties.
Specific Impulse
LOX/LCH4 engines typically achieve a specific impulse of around 350 seconds in vacuum, offering higher efficiency compared to LOX/RP-1 engines but lower than LOX/LH2 configurations.
Cryogenic Propellants
LOX offers higher density and easier storage while LCH4 provides higher specific impulse and cleaner combustion, making each cryogenic propellant suitable for different aerospace propulsion applications.
Oxidizer-Fuel Ratio
The optimal oxidizer-to-fuel ratio (O/F) for LOX (liquid oxygen) and LCH4 (liquid methane) combustion typically ranges between 3.5:1 to 3.8:1, maximizing specific impulse and combustion efficiency.
Regenerative Cooling
LOX/kerosene engines require more complex regenerative cooling systems due to higher combustion temperatures compared to LOX/LCH4 engines, which benefit from methane's higher heat capacity and cleaner combustion for more efficient and durable cooling channels.
Coking Resistance
Liquid Oxygen (LOX) combined with LNG (LCH4) fuels exhibits higher coking resistance due to cleaner combustion and lower carbon deposition compared to hydrocarbon-rich propellants.
Engine Cycle Efficiency
LOX/LCH4 engines achieve higher cycle efficiency than LOX/RP-1 due to methane's higher specific impulse and cleaner combustion reducing engine deposits.
Methalox Engines
Methalox engines utilizing liquid methane and liquid oxygen offer higher specific impulse and cleaner combustion compared to traditional LOX/RP-1 engines, making them ideal for reusable rocket stages and deep-space missions.
Boil-Off Management
LOX exhibits lower boil-off rates compared to LCH4 due to its higher boiling point, making it more efficient for long-term cryogenic storage and boil-off management.
Turbopump Compatibility
Turbopump compatibility with LOX supports higher rotational speeds due to its cryogenic properties, whereas LCH4 turbopumps require materials resistant to methane embrittlement and typically operate at lower temperatures and pressures.
Clean Combustion
LOX and LCH4 offer cleaner combustion with LCH4 producing fewer pollutants due to its higher hydrogen-to-carbon ratio and lower soot formation compared to LOX.
LOX vs LCH4 Infographic
 njnir.com
njnir.com