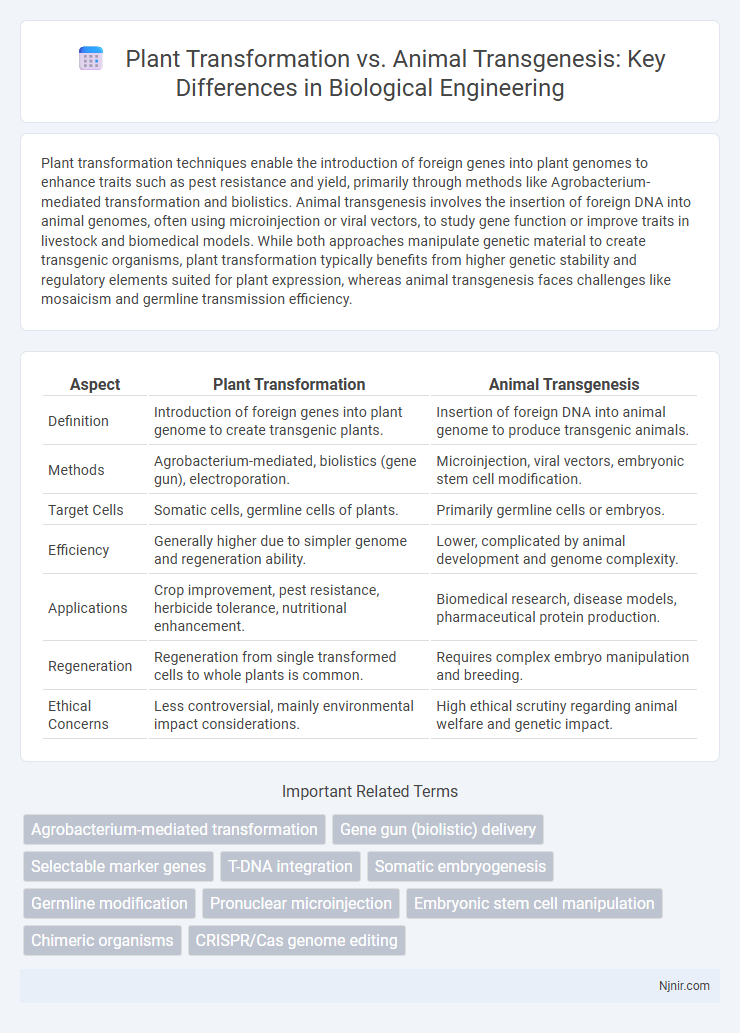
Plant transformation techniques enable the introduction of foreign genes into plant genomes to enhance traits such as pest resistance and yield, primarily through methods like Agrobacterium-mediated transformation and biolistics. Animal transgenesis involves the insertion of foreign DNA into animal genomes, often using microinjection or viral vectors, to study gene function or improve traits in livestock and biomedical models. While both approaches manipulate genetic material to create transgenic organisms, plant transformation typically benefits from higher genetic stability and regulatory elements suited for plant expression, whereas animal transgenesis faces challenges like mosaicism and germline transmission efficiency.
Table of Comparison
| Aspect | Plant Transformation | Animal Transgenesis |
|---|---|---|
| Definition | Introduction of foreign genes into plant genome to create transgenic plants. | Insertion of foreign DNA into animal genome to produce transgenic animals. |
| Methods | Agrobacterium-mediated, biolistics (gene gun), electroporation. | Microinjection, viral vectors, embryonic stem cell modification. |
| Target Cells | Somatic cells, germline cells of plants. | Primarily germline cells or embryos. |
| Efficiency | Generally higher due to simpler genome and regeneration ability. | Lower, complicated by animal development and genome complexity. |
| Applications | Crop improvement, pest resistance, herbicide tolerance, nutritional enhancement. | Biomedical research, disease models, pharmaceutical protein production. |
| Regeneration | Regeneration from single transformed cells to whole plants is common. | Requires complex embryo manipulation and breeding. |
| Ethical Concerns | Less controversial, mainly environmental impact considerations. | High ethical scrutiny regarding animal welfare and genetic impact. |
Overview of Plant Transformation and Animal Transgenesis
Plant transformation involves the stable introduction of foreign genes into plant genomes, commonly using methods such as Agrobacterium-mediated transformation and biolistics, to improve traits like pest resistance and stress tolerance. Animal transgenesis entails the integration of foreign DNA into animal genomes, often through techniques like microinjection, retroviral infection, and CRISPR-Cas9, to study gene function or develop models for human diseases. Both processes enable genetic modifications but differ significantly in methodology, target organisms, and applications within biotechnology and genetic research.
Historical Development and Milestones
Plant transformation began in the 1980s with the introduction of Agrobacterium tumefaciens-mediated gene transfer, marking a breakthrough in genetic engineering. Key milestones include the first transgenic tobacco plant in 1983 and the development of glyphosate-resistant crops in the 1990s, revolutionizing agriculture. In contrast, animal transgenesis advanced with microinjection techniques for mice in 1974, followed by cloning of Dolly the sheep in 1996, highlighting significant progress in mammalian genetic modification.
Core Techniques in Plant Transformation
Agrobacterium-mediated transformation remains a primary core technique in plant transformation due to its efficiency in transferring T-DNA into plant genomes. Particle bombardment, or biolistics, serves as an alternative method, physically delivering DNA-coated microprojectiles into plant cells, especially useful for monocots. Both methods aim to integrate foreign genes stably into the plant genome to create genetically modified crops with desired traits such as pest resistance or improved yield.
Primary Methods for Animal Transgenesis
Animal transgenesis primarily relies on microinjection of DNA into fertilized eggs, retroviral vector-mediated gene transfer, and the use of embryonic stem cell-mediated gene targeting. Each method offers distinct advantages: microinjection allows direct integration of genetic material, retroviral vectors enable efficient gene delivery with integration into host genomes, and embryonic stem cell techniques facilitate precise gene modifications through homologous recombination. These primary approaches contrast with plant transformation methods that often utilize Agrobacterium-mediated transfer or biolistics for gene insertion.
Genetic Vectors and Delivery Systems
Plant transformation primarily employs Agrobacterium-mediated vectors and biolistic particle delivery systems to introduce foreign DNA into plant cells, leveraging the natural DNA transfer capability of Agrobacterium tumefaciens or physical bombardment of cell tissues. Animal transgenesis commonly utilizes viral vectors such as lentiviruses or retroviruses and microinjection techniques to deliver genetic material directly into zygotes or embryos, enabling stable incorporation into the germline. The efficiency and specificity of genetic vectors and delivery systems differ significantly due to cellular structure variations and organismal developmental processes in plants versus animals.
Efficiency and Stability of Gene Integration
Plant transformation frequently utilizes Agrobacterium-mediated methods or biolistics, offering high efficiency and stable gene integration into the nuclear genome, resulting in heritable traits across generations. Animal transgenesis often relies on microinjection or viral vectors, which can lead to variable integration efficiency and mosaicism, impacting the uniformity and stability of transgene expression. Stability in plants is generally enhanced by precise insertion and expression control, whereas animals may require extensive screening to ensure consistent transgene integration and expression in germline cells.
Regulatory and Biosafety Considerations
Plant transformation and animal transgenesis face distinct regulatory frameworks and biosafety considerations due to differences in ecological impact and gene flow potential. Regulatory agencies like the USDA and EPA impose stringent risk assessments on genetically modified plants to prevent cross-contamination with native flora, while animal transgenesis oversight often involves FDA evaluation focused on animal welfare, food safety, and potential impacts on ecosystems. Biosafety protocols for plant transformation emphasize containment strategies and monitoring for horizontal gene transfer, whereas animal transgenesis requires ethical reviews and containment practices to avoid unintended effects on animal populations and biodiversity.
Ethical Implications in Plants and Animals
Ethical implications in plant transformation mainly revolve around biodiversity loss, potential gene flow to wild relatives, and long-term ecological impacts. In animal transgenesis, concerns intensify due to animal welfare, potential suffering, and moral considerations regarding the modification of sentient beings. Regulatory frameworks often impose stricter guidelines on animal transgenesis reflecting higher ethical scrutiny compared to plant genetic modifications.
Applications in Agriculture and Medicine
Plant transformation techniques enable the development of genetically modified crops with enhanced yield, pest resistance, and stress tolerance, significantly improving agricultural productivity and food security. Animal transgenesis facilitates the production of genetically engineered livestock with traits such as disease resistance and faster growth, as well as the creation of animal models for studying human diseases and developing novel medical therapies. Both technologies play critical roles in advancing biotechnology, with plant transformation primarily driving agricultural innovation and animal transgenesis contributing to biomedical research and pharmaceutical production.
Future Directions and Emerging Technologies
Future directions in plant transformation involve CRISPR-Cas9 base editing and prime editing techniques to enhance precision and efficiency in crop improvement. In animal transgenesis, advances such as somatic cell nuclear transfer combined with gene editing accelerate the development of disease-resistant and genetically enhanced livestock. Emerging technologies like nanotechnology-assisted gene delivery and synthetic biology hold transformative potential by improving gene integration and expression control in both plants and animals.
Agrobacterium-mediated transformation
Agrobacterium-mediated transformation is a highly efficient and widely used method for introducing foreign genes into plants by exploiting the natural DNA transfer capabilities of Agrobacterium tumefaciens, while animal transgenesis typically relies on microinjection or viral vectors to integrate transgenes into animal genomes.
Gene gun (biolistic) delivery
Gene gun (biolistic) delivery in plant transformation efficiently penetrates cell walls to introduce DNA directly into plant tissues, whereas its use in animal transgenesis is limited due to differences in cell structure and lower transformation efficiency compared to viral or microinjection methods.
Selectable marker genes
Selectable marker genes in plant transformation commonly include antibiotic resistance genes such as nptII and bar, while animal transgenesis frequently uses fluorescent proteins like GFP to identify successful genetic integration.
T-DNA integration
T-DNA integration during plant transformation typically involves site-specific insertion mediated by Agrobacterium tumefaciens, whereas animal transgenesis employs random integration methods such as microinjection or viral vectors resulting in less controlled genomic insertions.
Somatic embryogenesis
Somatic embryogenesis in plant transformation enables efficient clonal propagation and genetic modification by inducing embryo development from somatic cells, contrasting with animal transgenesis which relies primarily on germline modification techniques for stable transgene integration.
Germline modification
Plant transformation primarily involves stable integration of foreign DNA into the germline via techniques like Agrobacterium-mediated transformation or biolistics, enabling heritable traits, whereas animal transgenesis achieves germline modification through microinjection, viral vectors, or CRISPR-Cas9 to ensure transgene transmission across generations.
Pronuclear microinjection
Pronuclear microinjection is a widely used technique in animal transgenesis involving direct DNA injection into the zygote nucleus, whereas plant transformation primarily relies on Agrobacterium-mediated gene transfer or biolistics due to differences in cellular structure and regeneration processes.
Embryonic stem cell manipulation
Embryonic stem cell manipulation enables precise genetic modifications in animal transgenesis by targeting pluripotent cells, whereas plant transformation primarily relies on methods like Agrobacterium-mediated gene transfer or biolistics to introduce transgenes without utilizing embryonic stem cells.
Chimeric organisms
Chimeric organisms in plant transformation often arise through merging distinct cell lineages during tissue culture, whereas animal transgenesis chimeras result from integrating transgenes at embryonic stages to produce mosaic gene expression.
CRISPR/Cas genome editing
CRISPR/Cas genome editing enables precise gene modifications in plant transformation to improve traits like resistance and yield, while in animal transgenesis, it facilitates targeted gene insertion or knockout for disease modeling and agricultural advancements.
Plant transformation vs Animal transgenesis Infographic
 njnir.com
njnir.com