Hydrogel scaffolds offer a highly biocompatible environment that supports cell adhesion and proliferation, making them ideal for tissue engineering applications. 3D bioprinting enables precise spatial control over scaffold architecture and cell placement, enhancing the complexity and functionality of engineered tissues. Combining hydrogel scaffolds with 3D bioprinting technology advances regenerative medicine by creating more accurate tissue models and improving integration with host tissues.
Table of Comparison
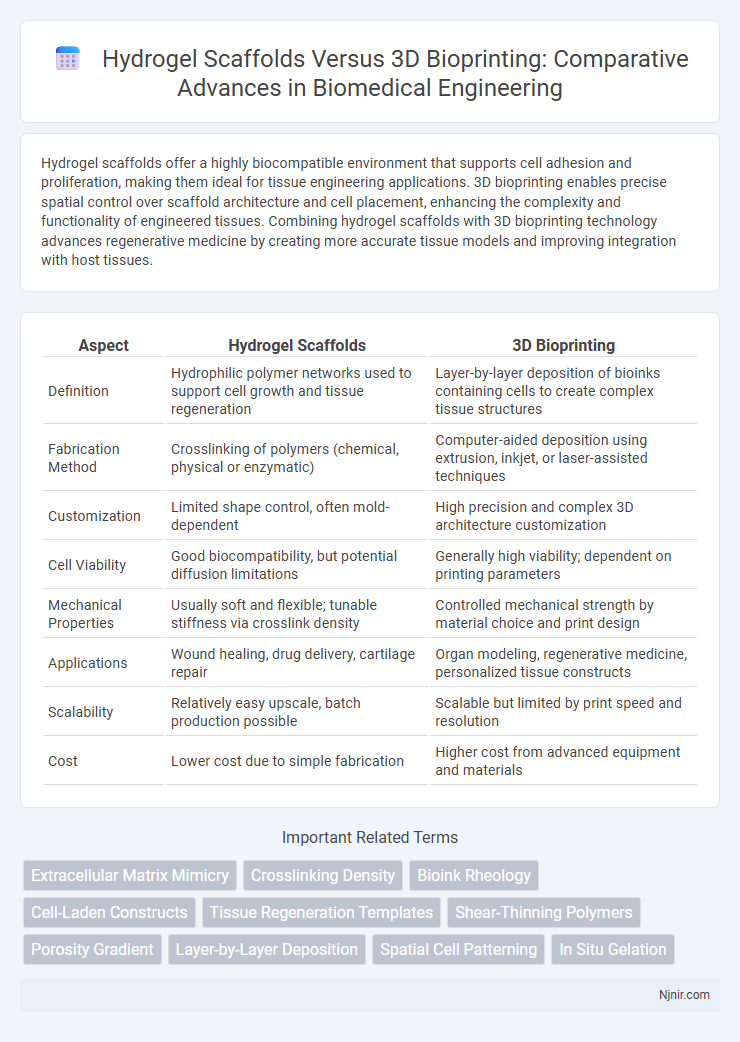
| Aspect | Hydrogel Scaffolds | 3D Bioprinting |
|---|---|---|
| Definition | Hydrophilic polymer networks used to support cell growth and tissue regeneration | Layer-by-layer deposition of bioinks containing cells to create complex tissue structures |
| Fabrication Method | Crosslinking of polymers (chemical, physical or enzymatic) | Computer-aided deposition using extrusion, inkjet, or laser-assisted techniques |
| Customization | Limited shape control, often mold-dependent | High precision and complex 3D architecture customization |
| Cell Viability | Good biocompatibility, but potential diffusion limitations | Generally high viability; dependent on printing parameters |
| Mechanical Properties | Usually soft and flexible; tunable stiffness via crosslink density | Controlled mechanical strength by material choice and print design |
| Applications | Wound healing, drug delivery, cartilage repair | Organ modeling, regenerative medicine, personalized tissue constructs |
| Scalability | Relatively easy upscale, batch production possible | Scalable but limited by print speed and resolution |
| Cost | Lower cost due to simple fabrication | Higher cost from advanced equipment and materials |
Introduction to Biomedical Engineering Innovations
Hydrogel scaffolds provide a biocompatible, hydrated matrix that mimics natural extracellular environments, facilitating cell growth and tissue regeneration in biomedical engineering. In contrast, 3D bioprinting offers precise spatial control by layering bioinks to construct complex, customizable tissue architectures with high resolution. These innovations revolutionize regenerative medicine by enabling tailored therapies and enhancing functional tissue repair outcomes.
Overview of Hydrogel Scaffolds
Hydrogel scaffolds are three-dimensional networks of hydrophilic polymers designed to mimic the extracellular matrix, providing a supportive environment for cell growth and tissue regeneration. Their unique water-retaining properties facilitate nutrient diffusion and waste removal, essential for maintaining cell viability in tissue engineering applications. Compared to 3D bioprinting, hydrogel scaffolds offer simplicity and tunable biochemical cues but may lack the structural precision and complexity achievable through advanced bioprinting techniques.
Fundamentals of 3D Bioprinting Technology
3D bioprinting technology utilizes layer-by-layer deposition of biomaterials, cells, and growth factors to fabricate complex tissue constructs with precise architectural control, distinguishing it from hydrogel scaffolds that serve primarily as static support matrices. The fundamentals of 3D bioprinting include bioink formulation, nozzle design, and printing parameters optimized to maintain cell viability and functionality during the printing process. This technology advances tissue engineering by enabling customizable scaffold geometries and heterogeneous cell distribution, surpassing the limitations of traditional hydrogel scaffolds.
Material Properties: Hydrogels in Tissue Engineering
Hydrogel scaffolds provide a highly porous and hydrated matrix that mimics the extracellular matrix, enabling cell adhesion, proliferation, and nutrient diffusion essential for tissue engineering. 3D bioprinting allows precise spatial control over hydrogel placement, enhancing structural complexity and mechanical properties tailored to specific tissue requirements. Material properties such as biocompatibility, biodegradability, and tunable stiffness in hydrogels critically influence cell behavior and the success of engineered tissues.
Fabrication Techniques: Traditional vs. Additive Manufacturing
Hydrogel scaffolds are traditionally fabricated using methods such as solvent casting, freeze-drying, and gas foaming, which offer control over porosity but lack precision in complex geometries. In contrast, 3D bioprinting employs additive manufacturing techniques like extrusion-based, inkjet, and laser-assisted printing, enabling layer-by-layer deposition with high spatial accuracy. This advanced fabrication allows customization of scaffold architecture and cell distribution, significantly enhancing tissue engineering outcomes compared to conventional hydrogel scaffold production.
Biocompatibility and Cellular Integration
Hydrogel scaffolds offer high biocompatibility due to their water-rich polymer networks that closely mimic the extracellular matrix, promoting cell adhesion and proliferation. In contrast, 3D bioprinting enables precise spatial control of multiple cell types and biomaterials, enhancing cellular integration and tissue complexity. Both approaches aim to optimize cellular microenvironments, but 3D bioprinting provides superior customization for heterogeneous tissue constructs.
Structural Complexity and Customization Capabilities
Hydrogel scaffolds provide a biocompatible matrix that mimics the extracellular environment, allowing for cellular growth but often lack the intricate architectural precision seen in native tissues. In contrast, 3D bioprinting enables precise spatial control and layer-by-layer construction, creating highly complex and customizable tissue structures tailored to specific anatomical requirements. Advanced bioprinting techniques facilitate the integration of multiple cell types and biomaterials, enhancing functional and structural fidelity beyond what traditional hydrogel scaffolds can achieve.
Clinical Applications: Hydrogel Scaffolds vs. 3D Bioprinting
Hydrogel scaffolds provide a biocompatible matrix that supports cell growth and tissue regeneration, making them widely used in wound healing, cartilage repair, and drug delivery systems. Conversely, 3D bioprinting offers precise spatial control to fabricate complex tissue structures, enhancing applications in personalized medicine, organ transplantation, and disease modeling. Both technologies are pivotal in regenerative medicine, with hydrogels offering simplicity and 3D bioprinting enabling customization for clinical applications.
Limitations and Challenges in Tissue Engineering
Hydrogel scaffolds face limitations such as poor mechanical strength and limited nutrient diffusion, which hinder effective cell proliferation and tissue integration. 3D bioprinting encounters challenges including bioink formulation complexity, scaffold fidelity, and scale-up difficulties for clinically relevant tissue sizes. Both methods require advanced vascularization strategies to overcome nutrient and oxygen delivery constraints for successful tissue engineering applications.
Future Perspectives and Emerging Trends
Hydrogel scaffolds offer a biocompatible matrix promoting cell proliferation and differentiation, while 3D bioprinting enables precise spatial control of multiple cell types and biomaterials, advancing personalized tissue engineering. Emerging trends focus on integrating smart hydrogels with stimuli-responsive properties and multi-material bioprinting techniques to enhance vascularization and functional tissue complexity. Future perspectives highlight the convergence of bioinks with advanced fabrication methods to create fully functional, implantable organs and improve regenerative medicine outcomes.
Extracellular Matrix Mimicry
Hydrogel scaffolds provide superior extracellular matrix mimicry compared to 3D bioprinting by offering enhanced biocompatibility and tunable physical properties that closely replicate native tissue environments.
Crosslinking Density
Hydrogel scaffolds with higher crosslinking density exhibit improved mechanical strength and stability compared to 3D bioprinted constructs, enhancing cell adhesion and tissue regeneration efficiency.
Bioink Rheology
Bioink rheology critically influences the printability and structural integrity of hydrogel scaffolds in 3D bioprinting by determining viscosity, shear-thinning behavior, and gelation kinetics.
Cell-Laden Constructs
Hydrogel scaffolds provide a supportive matrix for cell-laden constructs, while 3D bioprinting enables precise spatial arrangement and complex architectures for enhanced tissue engineering outcomes.
Tissue Regeneration Templates
Hydrogel scaffolds provide a biocompatible matrix mimicking extracellular environments for tissue regeneration, while 3D bioprinting enables precise spatial deposition of cells and biomaterials to create complex, customizable tissue templates.
Shear-Thinning Polymers
Shear-thinning polymers in hydrogel scaffolds enhance 3D bioprinting precision by enabling smooth extrusion and rapid structural recovery, improving cell viability and scaffold fidelity.
Porosity Gradient
Hydrogel scaffolds with controlled porosity gradients enhance nutrient diffusion and cell infiltration compared to uniform porosity in 3D bioprinting, improving tissue regeneration outcomes.
Layer-by-Layer Deposition
Layer-by-layer deposition in 3D bioprinting enables precise spatial control and complex tissue architecture formation, whereas hydrogel scaffolds primarily provide passive structural support for cell growth without intricate layering.
Spatial Cell Patterning
Hydrogel scaffolds enable precise spatial cell patterning by providing a biomimetic, tunable matrix, while 3D bioprinting offers advanced control over complex, heterogeneous cell distribution for tissue engineering applications.
In Situ Gelation
Hydrogel scaffolds enable in situ gelation by forming a supportive matrix directly at the target site, enhancing cell encapsulation and tissue integration compared to the layer-by-layer assembly typical of 3D bioprinting.
Hydrogel Scaffolds vs 3D Bioprinting Infographic
 njnir.com
njnir.com