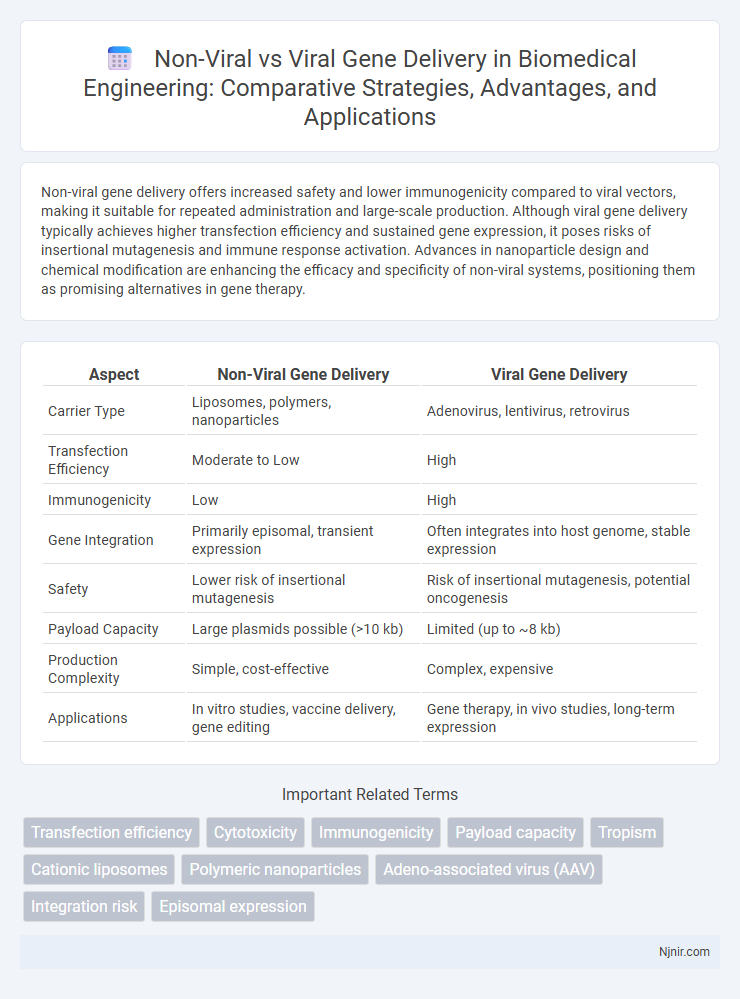
Non-viral gene delivery offers increased safety and lower immunogenicity compared to viral vectors, making it suitable for repeated administration and large-scale production. Although viral gene delivery typically achieves higher transfection efficiency and sustained gene expression, it poses risks of insertional mutagenesis and immune response activation. Advances in nanoparticle design and chemical modification are enhancing the efficacy and specificity of non-viral systems, positioning them as promising alternatives in gene therapy.
Table of Comparison
| Aspect | Non-Viral Gene Delivery | Viral Gene Delivery |
|---|---|---|
| Carrier Type | Liposomes, polymers, nanoparticles | Adenovirus, lentivirus, retrovirus |
| Transfection Efficiency | Moderate to Low | High |
| Immunogenicity | Low | High |
| Gene Integration | Primarily episomal, transient expression | Often integrates into host genome, stable expression |
| Safety | Lower risk of insertional mutagenesis | Risk of insertional mutagenesis, potential oncogenesis |
| Payload Capacity | Large plasmids possible (>10 kb) | Limited (up to ~8 kb) |
| Production Complexity | Simple, cost-effective | Complex, expensive |
| Applications | In vitro studies, vaccine delivery, gene editing | Gene therapy, in vivo studies, long-term expression |
Introduction to Gene Delivery Methods in Biomedical Engineering
Gene delivery methods in biomedical engineering are categorized into viral and non-viral systems, each with distinct mechanisms and applications for genetic material transfer. Viral gene delivery utilizes engineered viruses to exploit their natural ability to infect cells, achieving high transfection efficiency and stable gene expression, which is crucial for therapies targeting inherited disorders and cancer. Non-viral gene delivery employs physical or chemical techniques such as liposomes, nanoparticles, and electroporation, offering safer and less immunogenic alternatives, though typically with lower efficiency and transient gene expression.
Overview of Non-Viral Gene Delivery Systems
Non-viral gene delivery systems utilize synthetic or natural carriers such as liposomes, polymers, and nanoparticles to transport genetic material into target cells, offering safer profiles with lower immunogenicity compared to viral vectors. These systems enable scalable production and reduced risk of insertional mutagenesis, making them suitable for repetitive dosing in gene therapy. Challenges remain in enhancing transfection efficiency and targeted delivery, but advancements in material science and nanotechnology continue to improve the clinical potential of non-viral gene delivery methods.
Viral Gene Delivery: Mechanisms and Applications
Viral gene delivery utilizes engineered viruses, such as adenoviruses, lentiviruses, and adeno-associated viruses, to introduce genetic material into host cells by exploiting their natural infection mechanisms. These vectors efficiently transduce target cells through receptor-mediated endocytosis, nuclear entry, and integration or episomal maintenance of the delivered genes, enabling robust and sustained gene expression. Applications of viral gene delivery span gene therapy for inherited disorders, cancer immunotherapy, and vaccine development, offering precise targeting and high transduction efficiency compared to non-viral methods.
Efficiency Comparison: Non-Viral vs Viral Gene Delivery
Viral gene delivery systems, such as lentiviruses and adenoviruses, exhibit higher transfection efficiency due to their natural ability to infect host cells and integrate genetic material. Non-viral gene delivery methods, including liposomes and polymer-based vectors, show lower efficiency but offer improved safety profiles and reduced immunogenicity. Despite the lower transgene expression levels, ongoing advancements in non-viral vector design aim to narrow the efficiency gap while minimizing cytotoxic effects.
Safety Profiles: Immunogenicity and Toxicity Concerns
Non-viral gene delivery methods exhibit lower immunogenicity and reduced toxicity compared to viral vectors, making them safer for repeated administrations and minimizing adverse immune responses. Viral gene delivery systems, particularly adenoviruses and lentiviruses, often trigger strong immune reactions and potential insertional mutagenesis, raising safety concerns in clinical applications. Advances in synthetic vectors and nanoparticle formulations aim to maximize transfection efficiency while maintaining favorable safety profiles, addressing toxicity and immunogenicity challenges inherent to viral delivery.
Targeting Specificity in Gene Delivery Approaches
Non-viral gene delivery methods offer lower immunogenicity and enhanced safety but often lack the targeting specificity achieved by viral vectors, which efficiently exploit natural cell entry mechanisms to deliver genetic material to specific cell types. Viral gene delivery systems, such as lentiviruses and adeno-associated viruses, demonstrate high targeting precision due to engineered surface proteins that recognize specific cell receptors, facilitating selective transduction. Enhancements in non-viral carriers, including ligand-receptor binding and nanoparticle surface modifications, aim to improve targeting specificity, yet they remain less precise compared to the inherently evolved mechanisms present in viral vectors.
Scalability and Manufacturing for Clinical Applications
Non-viral gene delivery systems offer scalable production due to simpler manufacturing processes, lower biosafety risks, and ease of standardization, making them suitable for large-scale clinical applications. Viral gene delivery, while highly efficient, presents challenges in scalability because of complex vector production, rigorous purification requirements, and stringent regulatory oversight. Scalability and manufacturing constraints significantly influence clinical translation timelines, with non-viral methods currently providing more flexibility for mass production and consistent batch quality.
Recent Advances in Non-Viral Vectors
Recent advances in non-viral gene delivery systems have enhanced safety and efficiency by employing lipid nanoparticles, polymer-based carriers, and dendrimers to overcome cellular barriers and reduce immune responses. Innovations like CRISPR-Cas9 encapsulation and targeted ligands improve site-specific gene editing while minimizing off-target effects compared to viral vectors such as adenoviruses or lentiviruses. These developments address the limitations of viral gene delivery, including insertional mutagenesis and immunogenicity, positioning non-viral vectors as a promising alternative for clinical gene therapy applications.
Clinical Trials and Therapeutic Outcomes
Non-viral gene delivery systems have demonstrated improved safety profiles and lower immunogenicity in clinical trials compared to viral vectors, which often face challenges related to immune responses and insertional mutagenesis. Therapeutic outcomes with viral gene delivery have shown higher gene transfer efficiency and sustained expression, particularly in conditions like hemophilia and inherited retinal diseases, leading to significant clinical improvements. Emerging data suggest that non-viral methods, including lipid nanoparticles and electroporation, are gaining traction due to better scalability and reduced adverse events, although they currently exhibit lower transfection efficiency than viral approaches.
Future Directions in Gene Delivery Technologies
Future directions in gene delivery technologies emphasize enhancing the safety and efficiency of non-viral gene delivery systems, including advanced nanoparticle formulations and targeted lipid-based carriers to overcome cellular barriers. Novel viral vector engineering aims to minimize immunogenicity and increase tissue specificity through precise capsid modifications and synthetic biology approaches. Integration of CRISPR and other gene-editing tools with optimized delivery platforms promises to revolutionize personalized medicine by enabling precise and controlled genetic interventions.
Transfection efficiency
Viral gene delivery typically achieves higher transfection efficiency than non-viral methods due to its ability to effectively penetrate cell membranes and facilitate stable gene integration.
Cytotoxicity
Non-viral gene delivery exhibits significantly lower cytotoxicity compared to viral gene delivery, making it a safer alternative for therapeutic applications.
Immunogenicity
Non-viral gene delivery exhibits significantly lower immunogenicity compared to viral gene delivery, reducing the risk of immune responses and enhancing safety in gene therapy applications.
Payload capacity
Non-viral gene delivery systems typically offer larger payload capacities compared to viral vectors, which are limited by their capsid sizes, making non-viral methods advantageous for delivering large or multiple genes.
Tropism
Viral gene delivery exhibits high tropism by targeting specific cell types through natural receptor binding, whereas non-viral gene delivery lacks intrinsic tropism and often requires chemical modifications or targeting ligands to achieve cell-specific transfection.
Cationic liposomes
Cationic liposomes enhance non-viral gene delivery efficiency by facilitating DNA encapsulation and cellular uptake while minimizing immunogenicity compared to viral vectors.
Polymeric nanoparticles
Polymeric nanoparticles in non-viral gene delivery offer enhanced biocompatibility, lower immunogenicity, and customizable surface modifications compared to viral vectors, making them a safer and more versatile option for targeted gene therapy.
Adeno-associated virus (AAV)
Adeno-associated virus (AAV) viral gene delivery offers higher transduction efficiency and longer-term gene expression compared to non-viral gene delivery methods, which provide safer profiles and larger gene cargo capacity but lower efficiency.
Integration risk
Non-viral gene delivery methods exhibit a significantly lower genomic integration risk compared to viral gene delivery, reducing potential insertional mutagenesis and enhancing safety profiles for therapeutic applications.
Episomal expression
Non-viral gene delivery offers safer episomal expression with lower immunogenicity but typically results in transient gene expression, whereas viral gene delivery achieves higher efficiency and longer-lasting episomal presence at the risk of immunogenic responses.
Non-viral gene delivery vs Viral gene delivery Infographic
 njnir.com
njnir.com