Tissue engineering involves creating functional tissues by combining scaffolds, cells, and bioactive molecules to restore or replace damaged tissues, while organoid culture enables the development of miniature, self-organizing, three-dimensional cell structures that mimic organ functions for disease modeling and drug testing. Tissue engineering emphasizes scaffold architecture and mechanical properties to guide cell growth and tissue formation, whereas organoid culture relies on stem cell differentiation within a controlled microenvironment without external scaffolds. Both approaches offer complementary advantages in regenerative medicine, with tissue engineering facilitating transplantation and organoids providing advanced platforms for personalized medicine.
Table of Comparison
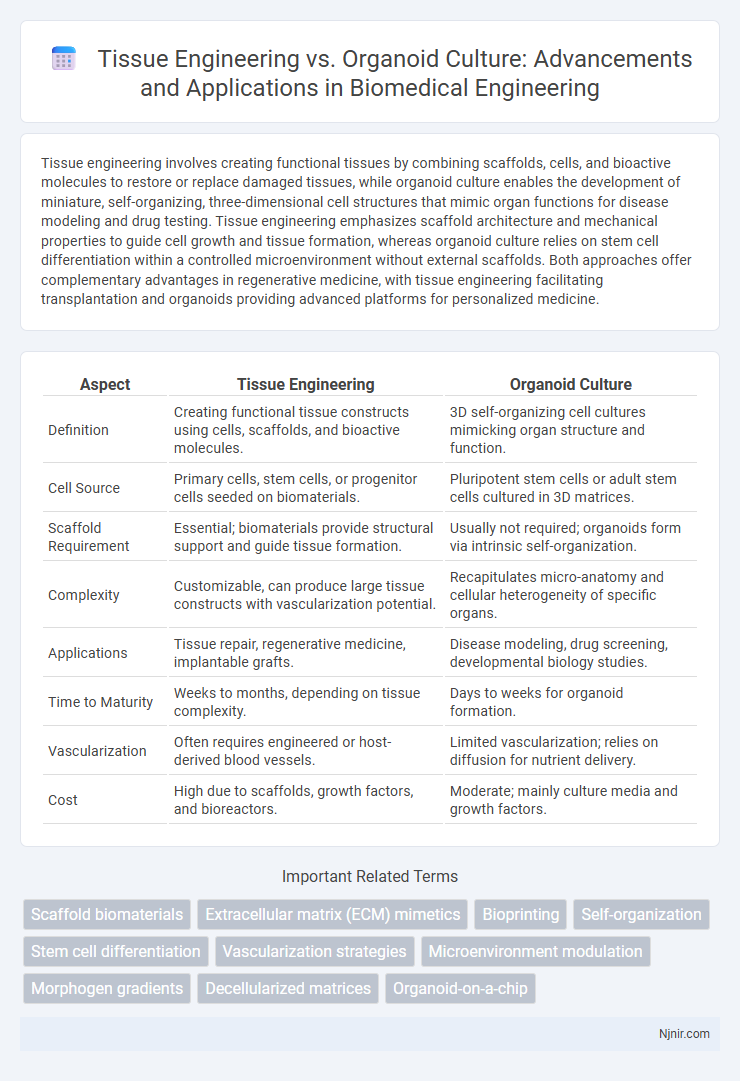
| Aspect | Tissue Engineering | Organoid Culture |
|---|---|---|
| Definition | Creating functional tissue constructs using cells, scaffolds, and bioactive molecules. | 3D self-organizing cell cultures mimicking organ structure and function. |
| Cell Source | Primary cells, stem cells, or progenitor cells seeded on biomaterials. | Pluripotent stem cells or adult stem cells cultured in 3D matrices. |
| Scaffold Requirement | Essential; biomaterials provide structural support and guide tissue formation. | Usually not required; organoids form via intrinsic self-organization. |
| Complexity | Customizable, can produce large tissue constructs with vascularization potential. | Recapitulates micro-anatomy and cellular heterogeneity of specific organs. |
| Applications | Tissue repair, regenerative medicine, implantable grafts. | Disease modeling, drug screening, developmental biology studies. |
| Time to Maturity | Weeks to months, depending on tissue complexity. | Days to weeks for organoid formation. |
| Vascularization | Often requires engineered or host-derived blood vessels. | Limited vascularization; relies on diffusion for nutrient delivery. |
| Cost | High due to scaffolds, growth factors, and bioreactors. | Moderate; mainly culture media and growth factors. |
Introduction to Tissue Engineering and Organoid Culture
Tissue engineering involves the use of scaffolds, cells, and biologically active molecules to create functional tissues for regenerative medicine, relying on biomaterials to guide cell growth and differentiation. Organoid culture utilizes stem cells to self-organize into three-dimensional structures that mimic the architecture and function of native organs, providing advanced models for studying development and disease. Both techniques offer complementary approaches in biomedical research, with tissue engineering focusing on creating transplantable tissues and organoid culture emphasizing in vitro modeling of organ function.
Fundamental Principles: Tissue Engineering Explained
Tissue engineering involves the integration of cells, scaffolds, and bioactive molecules to develop functional tissues that restore, maintain, or improve biological functions. It relies on biomaterials engineered to mimic the extracellular matrix, supporting cell adhesion, proliferation, and differentiation under controlled biochemical and mechanical environments. Unlike organoid culture, which self-organizes cells into microtissues, tissue engineering emphasizes the design of three-dimensional constructs with precise architecture for clinical transplantation and regenerative medicine.
Organoid Culture: Defining Characteristics and Methods
Organoid culture involves the growth of three-dimensional, miniaturized, and simplified versions of organs derived from stem cells, exhibiting self-organization and differentiation that mimic native tissue architecture and function. Key methods include embedding stem cells in an extracellular matrix like Matrigel, supplying a defined cocktail of growth factors, and maintaining conditions that promote cellular heterogeneity and morphogenetic processes. These characteristics make organoid culture a powerful tool for disease modeling, drug screening, and personalized medicine, distinct from traditional tissue engineering which often relies on scaffold-based constructs and larger-scale tissue fabrication.
Biomaterials and Scaffolds in Tissue Engineering
Biomaterials and scaffolds in tissue engineering provide structural support and a microenvironment that mimics native extracellular matrix, promoting cell adhesion, proliferation, and differentiation. These scaffolds are typically made from biocompatible polymers like polylactic acid (PLA), polycaprolactone (PCL), or natural proteins such as collagen and gelatin, enabling controlled mechanical properties and degradation rates tailored to specific tissue types. Unlike organoid culture, which relies on self-organizing stem cells in 3D matrices like Matrigel, tissue engineering's scaffold-based approach offers precise spatial control for tissue architecture and vascularization.
Cellular Sources and Differentiation in Organoid Culture
Organoid culture relies primarily on pluripotent stem cells (PSCs) or adult stem cells as cellular sources, enabling the self-organization and differentiation into complex, three-dimensional mini-organs that recapitulate in vivo tissue architecture and function. Differentiation in organoid culture is directed by carefully controlled biochemical cues and signaling pathways, such as Wnt, Notch, and BMP, which mimic developmental processes and promote lineage-specific cell fate decisions. This intrinsic capacity for self-organization and multilineage differentiation distinguishes organoid culture from traditional tissue engineering, which often requires pre-fabricated scaffolds and extrinsic cell seeding strategies.
Applications in Disease Modeling and Regenerative Medicine
Tissue engineering utilizes biomaterials and stem cells to create functional tissue constructs for regenerative medicine, enabling organ replacement and wound healing applications. Organoid culture involves the 3D growth of stem cell-derived mini-organs that mimic native tissue architecture and function, providing advanced models for disease modeling and drug testing. Both approaches revolutionize personalized medicine by offering platforms to study complex pathologies and develop targeted therapies.
Advantages and Limitations: Tissue Engineering vs. Organoid Culture
Tissue engineering offers precise control over the microenvironment and scaffold design, enabling the creation of tissue constructs with defined architecture and mechanical properties, but it faces challenges in replicating complex cell-cell interactions seen in vivo. Organoid culture excels in mimicking native tissue organization and cellular diversity through self-organization processes, providing physiologically relevant models, yet it suffers from variability, limited scalability, and difficulties in vascularization. Both approaches complement each other by balancing structural control and biological complexity, but limitations in nutrient diffusion and long-term viability remain critical hurdles.
Recent Advances and Breakthroughs
Recent advances in tissue engineering have harnessed 3D bioprinting and advanced biomaterials to create functional tissues with enhanced vascularization and mechanical properties, enabling more accurate disease models and potential therapeutic applications. Organoid culture breakthroughs include improved differentiation protocols and organoid-on-a-chip systems, allowing higher fidelity recapitulation of organ complexity and dynamic microenvironments for drug testing and personalized medicine. Integration of organoid technologies with tissue engineering scaffolds is emerging, facilitating more physiologically relevant tissue constructs and accelerating translational research in regenerative medicine.
Translational Challenges and Future Perspectives
Tissue engineering faces translational challenges such as vascularization, immune response integration, and scalability, which hinder the clinical application of engineered tissues. Organoid culture offers promising disease modeling and personalized medicine opportunities but struggles with replicating complex tissue architecture and long-term functionality in vivo. Future perspectives emphasize combining the strengths of both approaches to enhance regenerative medicine, improve drug testing platforms, and achieve more effective patient-specific therapies.
Ethical and Regulatory Considerations
Tissue engineering faces stringent ethical and regulatory scrutiny due to its use of stem cells and potential for creating implantable human tissues, requiring compliance with FDA guidelines and institutional review boards to ensure patient safety and consent. Organoid culture, while also involving human-derived materials, encounters fewer regulatory hurdles as it is primarily used for in vitro disease modeling and drug testing without direct implantation into patients. Both fields must navigate concerns around genetic manipulation, data privacy, and equitable access to emerging technologies to align with bioethical standards and public trust.
Scaffold biomaterials
Scaffold biomaterials in tissue engineering provide structural support and promote cell growth, whereas organoid culture relies on self-organizing cellular aggregates often embedded in extracellular matrix-based hydrogels for 3D tissue modeling.
Extracellular matrix (ECM) mimetics
Tissue engineering employs synthetic and natural ECM mimetics to provide structural support and biochemical cues for cell growth, while organoid culture relies on ECM-derived hydrogels like Matrigel to recreate a niche that promotes self-organized tissue development.
Bioprinting
Bioprinting enhances tissue engineering by precisely fabricating complex 3D structures using living cells, while organoid culture relies on self-organized cell clusters to model organ function without external patterning.
Self-organization
Self-organization in organoid culture enables reproducible, multicellular structures mimicking native tissue architecture, while tissue engineering often relies on scaffold-guided assembly limiting autonomous spatial organization.
Stem cell differentiation
Stem cell differentiation in tissue engineering enables scalable, structured tissue formation, whereas organoid culture promotes self-organized, functional mini-organs mimicking in vivo environments.
Vascularization strategies
Tissue engineering employs scaffold-based methods and growth factor delivery for vascularization, while organoid culture relies on co-culture systems and microfluidic devices to enhance vascular network formation.
Microenvironment modulation
Microenvironment modulation in tissue engineering involves precise control of biochemical and mechanical cues to promote tissue regeneration, whereas organoid culture relies on self-organizing stem cells within a defined niche to mimic organ-specific microenvironments.
Morphogen gradients
Morphogen gradients in tissue engineering provide controlled spatial cues for cell differentiation, whereas organoid culture relies on self-organizing cells responding to endogenous morphogen gradients to mimic tissue architecture.
Decellularized matrices
Decellularized matrices in tissue engineering provide native extracellular framework promoting cell adhesion and tissue regeneration, whereas organoid culture relies on synthetic or natural hydrogels to mimic microenvironments for 3D cellular self-organization and functional organ-like structures.
Organoid-on-a-chip
Organoid-on-a-chip technology integrates organoid culture with microfluidic tissue engineering to enable precise control of the cellular microenvironment, enhancing disease modeling and drug testing accuracy.
tissue engineering vs organoid culture Infographic
 njnir.com
njnir.com