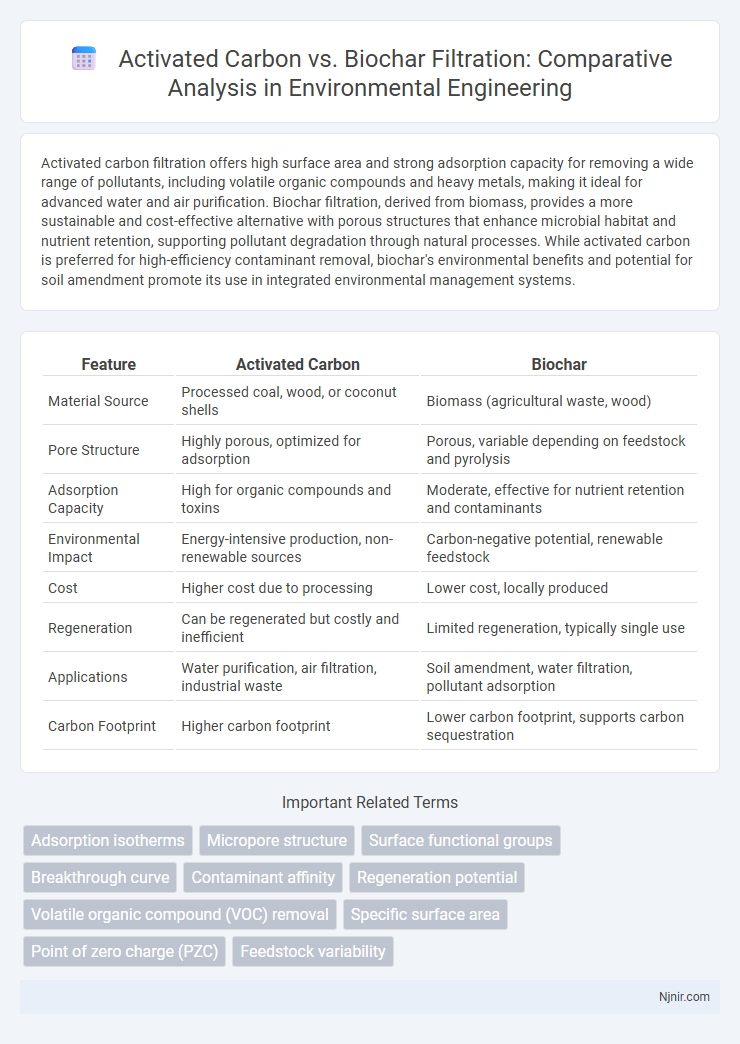
Activated carbon filtration offers high surface area and strong adsorption capacity for removing a wide range of pollutants, including volatile organic compounds and heavy metals, making it ideal for advanced water and air purification. Biochar filtration, derived from biomass, provides a more sustainable and cost-effective alternative with porous structures that enhance microbial habitat and nutrient retention, supporting pollutant degradation through natural processes. While activated carbon is preferred for high-efficiency contaminant removal, biochar's environmental benefits and potential for soil amendment promote its use in integrated environmental management systems.
Table of Comparison
| Feature | Activated Carbon | Biochar |
|---|---|---|
| Material Source | Processed coal, wood, or coconut shells | Biomass (agricultural waste, wood) |
| Pore Structure | Highly porous, optimized for adsorption | Porous, variable depending on feedstock and pyrolysis |
| Adsorption Capacity | High for organic compounds and toxins | Moderate, effective for nutrient retention and contaminants |
| Environmental Impact | Energy-intensive production, non-renewable sources | Carbon-negative potential, renewable feedstock |
| Cost | Higher cost due to processing | Lower cost, locally produced |
| Regeneration | Can be regenerated but costly and inefficient | Limited regeneration, typically single use |
| Applications | Water purification, air filtration, industrial waste | Soil amendment, water filtration, pollutant adsorption |
| Carbon Footprint | Higher carbon footprint | Lower carbon footprint, supports carbon sequestration |
Introduction to Activated Carbon and Biochar Filtration
Activated carbon and biochar are highly porous materials used in water and air filtration due to their large surface areas and strong adsorption capacities. Activated carbon is produced by heating carbon-rich materials like coal, wood, or coconut shells at high temperatures in an oxygen-limited environment, creating an extensive network of pores that trap contaminants. Biochar, derived from the pyrolysis of biomass such as agricultural waste, offers similar adsorption properties but also enhances soil quality and carbon sequestration, making it a sustainable alternative in filtration applications.
Material Origins: Activated Carbon and Biochar Explained
Activated carbon is derived from carbon-rich materials such as coconut shells, coal, or wood that undergo high-temperature activation in an oxygen-limited environment, enhancing its porous structure for increased adsorption capacity. Biochar, produced through the pyrolysis of biomass like agricultural waste or forestry residues in an oxygen-limited setting, retains more of the original organic material and mineral content, influencing its filtration characteristics. The distinct thermal processes and raw material sources create variations in surface area, pore size distribution, and chemical properties, directly impacting their effectiveness in contaminant removal.
Production Processes and Environmental Impact
Activated carbon is produced through high-temperature pyrolysis followed by activation using steam or chemicals, resulting in a highly porous material with a large surface area ideal for adsorption. Biochar is created via pyrolysis of biomass under limited oxygen conditions, primarily aimed at carbon sequestration but also serving as a filtration medium with lower adsorption capacity than activated carbon. The environmental impact of activated carbon production includes significant energy consumption and chemical use, whereas biochar production is generally more sustainable, utilizing waste biomass and enhancing soil health while reducing greenhouse gas emissions.
Physical and Chemical Properties Comparison
Activated carbon exhibits a highly porous structure with a surface area ranging from 500 to 1500 m2/g, featuring abundant micropores that enhance adsorption capacity for organic compounds. Biochar typically has a lower surface area, around 200 to 400 m2/g, with a more heterogeneous pore size distribution, including mesopores and macropores, which influences its adsorption and filtration efficiency differently. Chemically, activated carbon possesses a higher content of functional groups like carboxyl, hydroxyl, and carbonyl that facilitate chemical adsorption and catalytic reactions, whereas biochar contains more ash and mineral components, affecting its pH buffering capacity and nutrient retention in filtration applications.
Adsorption Mechanisms: How Do They Work?
Activated carbon filtration relies on its highly porous structure and large surface area, enabling the adsorption of contaminants through physical adsorption and chemisorption processes. Biochar filtration utilizes its porous matrix and surface functional groups to capture pollutants via adsorption and ion exchange mechanisms, often enhanced by biochar's natural affinity for organic compounds. Both materials operate by trapping molecules on their surfaces, but activated carbon typically exhibits higher adsorption capacity due to its engineered pore distribution and surface chemistry.
Pollutant Removal Efficiency: Side-by-Side Analysis
Activated carbon filtration demonstrates high pollutant removal efficiency, effectively adsorbing volatile organic compounds (VOCs), heavy metals, and chlorine from water and air due to its extensive surface area and porous structure. Biochar filtration, although less processed, offers comparable performance in removing organic pollutants and some heavy metals, benefiting from its renewable origin and capacity for nutrient retention. Side-by-side analysis reveals that activated carbon generally outperforms biochar in contaminant adsorption rates, but biochar's versatility and sustainability make it a competitive alternative in specific environmental applications.
Cost-Effectiveness and Economic Considerations
Activated carbon filtration offers high adsorption capacity but typically comes with increased production and regeneration costs, making it less cost-effective for large-scale or continuous use. Biochar filtration presents a more economical alternative due to lower raw material expenses and potential for local production, reducing transportation and manufacturing costs. Economic considerations favor biochar in sustainable and low-budget applications, whereas activated carbon remains preferable when maximum contaminant removal efficiency justifies higher expenditure.
Sustainability and Life Cycle Assessment
Activated carbon filtration demonstrates high adsorption efficiency but relies heavily on non-renewable resources and energy-intensive production processes, leading to a larger carbon footprint in life cycle assessments. Biochar filtration, derived from biomass pyrolysis, offers a renewable and carbon-neutral alternative with enhanced soil amendment benefits post-use, contributing to circular economy principles. Life cycle assessments emphasize biochar's lower environmental impact and sustainability potential due to reduced greenhouse gas emissions and resource regeneration capabilities.
Applications in Water and Air Filtration
Activated carbon exhibits exceptional adsorption capabilities for removing volatile organic compounds (VOCs), chlorine, and heavy metals from water and air, making it a preferred choice in municipal water treatment and industrial air purification. Biochar, derived from biomass pyrolysis, enhances microbial activity and nutrient retention in water filtration systems, offering sustainable solutions for agricultural runoff and wastewater management. Both materials contribute significantly to improving air quality by capturing pollutants, with activated carbon favored for high-efficiency filters and biochar gaining attention for eco-friendly, low-cost applications.
Future Prospects and Innovations in Filtration Technologies
Activated carbon filtration continues to advance with nano-engineered surfaces and enhanced adsorption capacities targeting emerging contaminants like pharmaceuticals and microplastics. Biochar filtration innovations harness its renewable feedstock origins, integrating biochar composites with photocatalytic materials for simultaneous contaminant degradation and adsorption. Future filtration technologies emphasize hybrid systems combining activated carbon's high surface area with biochar's sustainability to achieve superior water purification performance and environmental impact reduction.
Adsorption isotherms
Activated carbon exhibits higher adsorption capacity and faster kinetics compared to biochar, as demonstrated by Langmuir and Freundlich isotherms in water contaminant removal studies.
Micropore structure
Activated carbon exhibits a highly developed micropore structure with pore sizes typically below 2 nanometers, enabling superior adsorption efficiency compared to biochar, which generally has larger and less uniform pores, resulting in lower surface area and reduced contaminant retention capacity.
Surface functional groups
Activated carbon exhibits a higher density of diverse surface functional groups, including carboxyl, hydroxyl, and carbonyl moieties, enhancing its adsorption capacity compared to biochar, which typically contains fewer oxygen-containing groups due to lower activation temperatures.
Breakthrough curve
Biochar filtration demonstrates a slower breakthrough curve than activated carbon, indicating prolonged adsorption capacity and enhanced contaminant retention in water treatment applications.
Contaminant affinity
Activated carbon exhibits higher contaminant affinity due to its greater surface area and pore structure compared to biochar, making it more effective for removing organic pollutants and heavy metals in filtration applications.
Regeneration potential
Activated carbon exhibits higher regeneration potential through thermal or chemical treatments compared to biochar, which typically shows limited regeneration capacity due to its less uniform pore structure and lower stability.
Volatile organic compound (VOC) removal
Activated carbon filtration removes 90-99% of volatile organic compounds (VOCs) due to its high surface area and porous structure, while biochar filtration typically achieves 60-80% VOC removal, making activated carbon more effective for comprehensive VOC adsorption.
Specific surface area
Activated carbon filtration typically offers a higher specific surface area ranging from 500 to 1500 m2/g compared to biochar's 200 to 800 m2/g, enhancing its adsorption capacity for contaminants.
Point of zero charge (PZC)
Activated carbon typically has a Point of Zero Charge (PZC) around pH 6-7, while biochar's PZC varies widely from acidic to alkaline values depending on feedstock and pyrolysis conditions, significantly influencing their adsorption performance in water filtration.
Feedstock variability
Activated carbon filtration performance depends on consistent feedstock like coal or coconut shells, whereas biochar filtration efficiency varies significantly due to diverse biomass feedstocks affecting porosity and adsorption capacity.
activated carbon vs biochar filtration Infographic
 njnir.com
njnir.com