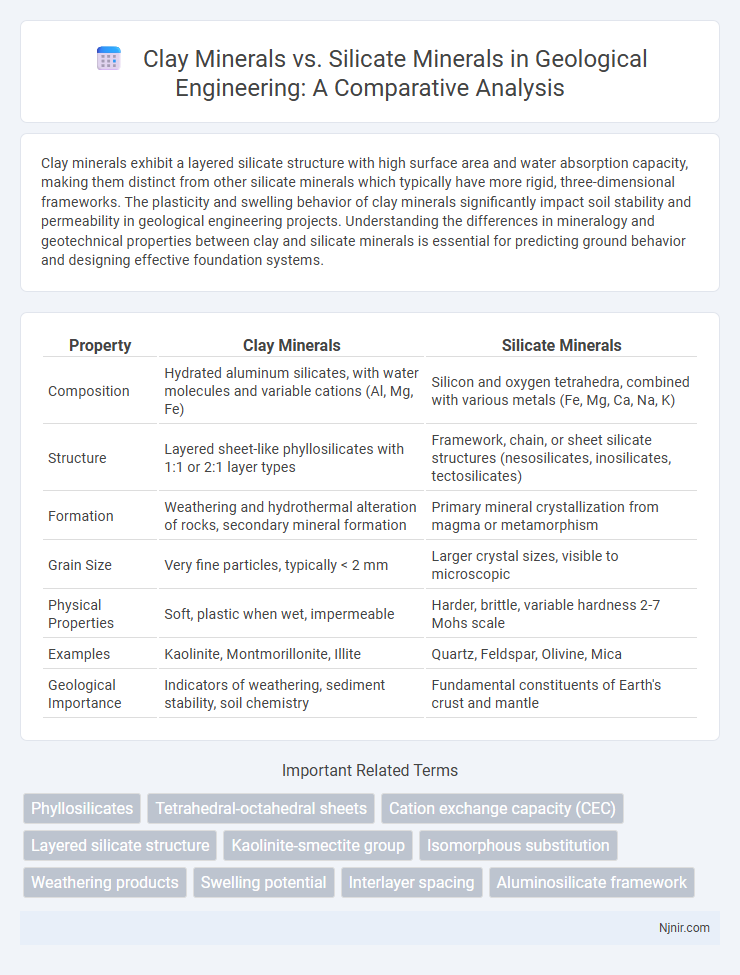
Clay minerals exhibit a layered silicate structure with high surface area and water absorption capacity, making them distinct from other silicate minerals which typically have more rigid, three-dimensional frameworks. The plasticity and swelling behavior of clay minerals significantly impact soil stability and permeability in geological engineering projects. Understanding the differences in mineralogy and geotechnical properties between clay and silicate minerals is essential for predicting ground behavior and designing effective foundation systems.
Table of Comparison
| Property | Clay Minerals | Silicate Minerals |
|---|---|---|
| Composition | Hydrated aluminum silicates, with water molecules and variable cations (Al, Mg, Fe) | Silicon and oxygen tetrahedra, combined with various metals (Fe, Mg, Ca, Na, K) |
| Structure | Layered sheet-like phyllosilicates with 1:1 or 2:1 layer types | Framework, chain, or sheet silicate structures (nesosilicates, inosilicates, tectosilicates) |
| Formation | Weathering and hydrothermal alteration of rocks, secondary mineral formation | Primary mineral crystallization from magma or metamorphism |
| Grain Size | Very fine particles, typically < 2 mm | Larger crystal sizes, visible to microscopic |
| Physical Properties | Soft, plastic when wet, impermeable | Harder, brittle, variable hardness 2-7 Mohs scale |
| Examples | Kaolinite, Montmorillonite, Illite | Quartz, Feldspar, Olivine, Mica |
| Geological Importance | Indicators of weathering, sediment stability, soil chemistry | Fundamental constituents of Earth's crust and mantle |
Introduction to Clay Minerals and Silicate Minerals
Clay minerals are a subgroup of silicate minerals characterized by their fine-grained, layered structure and significant role in soil formation and sedimentary processes. Silicate minerals, representing over 90% of the Earth's crust, consist of silicon-oxygen tetrahedra linked in various forms, including isolated, chain, sheet, and framework structures. The key difference lies in clay minerals' specific sheet silicate composition and their ability to absorb water and swell, which influences their physical and chemical properties compared to broader silicate mineral groups.
Chemical Composition: Clays vs. Silicates
Clay minerals are predominantly composed of hydrous aluminum phyllosilicates with varying amounts of magnesium and iron, characterized by layered structures containing silica tetrahedra and alumina octahedra. Silicate minerals encompass a broader group with a fundamental framework of silicon-oxygen tetrahedra that bond with various metal cations, such as aluminum, iron, calcium, and potassium. The chemical composition difference lies in clays having a pronounced presence of aluminum and water in their structure, while silicates exhibit diverse elemental combinations forming single, double, or framework tetrahedral arrangements.
Crystal Structure Differences
Clay minerals exhibit a layered crystal structure composed of tetrahedral silica sheets and octahedral alumina sheets, forming 1:1 or 2:1 phyllosilicate layers that promote high surface area and cation exchange capacity. Silicate minerals, in contrast, display a wide variety of crystal structures ranging from isolated tetrahedra in olivine to three-dimensional frameworks in quartz and feldspar, influencing their hardness and stability. The fundamental difference lies in the sheet-like arrangement of clay minerals versus the diverse three-dimensional or chain structures in other silicates, affecting their physical and chemical properties.
Formation Processes in Geological Settings
Clay minerals primarily form through the chemical weathering and hydrothermal alteration of silicate minerals in sedimentary and low-temperature geological environments. Silicate minerals crystallize from magma or metamorphose under high-temperature and high-pressure conditions within the Earth's crust. These differing formation processes result in clay minerals typically being fine-grained and secondary products, while silicate minerals represent primary crystalline phases in igneous and metamorphic rocks.
Physical Properties and Identification
Clay minerals, characterized by their fine-grained, platy morphology, exhibit low hardness, typically less than 2 on the Mohs scale, and display a greasy or soapy feel. Silicate minerals, encompassing a broader group including quartz and feldspar, have varied hardness from 6 to 7 and commonly show distinct crystal forms and cleavage patterns. Identification of clay minerals often relies on their plasticity, swelling behavior, and X-ray diffraction, while silicate minerals are identified through their crystal habit, hardness, and cleavage.
Role in Soil Mechanics and Engineering
Clay minerals, primarily composed of fine-grained hydrous aluminum phyllosilicates, significantly influence soil plasticity, compressibility, and permeability due to their sheet-like structure and high surface area. Silicate minerals, including quartz and feldspar, contribute mainly to soil texture and mechanical strength, providing mineral stability and resistance to weathering. The interaction between clay minerals' swelling properties and silicate minerals' granular framework determines soil behavior under load, critical in foundation design and slope stability analysis in geotechnical engineering.
Environmental Stability and Weathering
Clay minerals exhibit higher environmental stability and slower weathering rates compared to silicate minerals due to their fine-grained structure and strong interlayer bonding. Silicate minerals, particularly mafic and feldspathic types, weather rapidly under surface conditions releasing essential nutrients but leading to soil acidification and mineral depletion. The transformation of silicate minerals into clay minerals during weathering enhances soil fertility and contributes to long-term landscape evolution and carbon sequestration.
Industrial and Engineering Applications
Clay minerals, primarily composed of hydrous aluminum phyllosilicates, exhibit high plasticity, low permeability, and excellent adsorption properties, making them essential for ceramics, drilling muds, and sealants in civil engineering. Silicate minerals, such as quartz and feldspar, provide structural strength and durability, widely used in concrete production, glass manufacturing, and refractory materials. The distinct microstructures and chemical compositions of clay and silicate minerals dictate their specific roles in industrial processes and engineering applications, optimizing performance based on their mechanical and thermal properties.
Challenges in Geological Engineering Projects
Clay minerals exhibit high plasticity and water retention, causing unpredictable swelling and shrinkage that complicate foundation stability in geological engineering projects. Silicate minerals, while generally more stable, can undergo chemical weathering processes that alter soil composition and mechanical properties over time. Managing these variable mineral behaviors requires advanced site characterization and adaptive engineering solutions to ensure long-term structural integrity.
Future Research Trends in Clay and Silicate Minerals
Future research trends in clay and silicate minerals emphasize advanced characterization techniques such as synchrotron X-ray diffraction and atomic force microscopy to unravel nanoscale structures and interlayer chemistry. Innovations in computational modeling and machine learning are accelerating the prediction of mineral behavior under environmental stress and in industrial applications. Sustainable utilization and functionalization of clay and silicate minerals for environmental remediation, energy storage, and nanocomposite materials remain key focus areas driving interdisciplinary collaboration.
Phyllosilicates
Phyllosilicates, a subgroup of silicate minerals, are characterized by their sheet-like crystal structure and include important clay minerals such as kaolinite, illite, and smectite, which significantly influence soil properties and sedimentary geochemistry.
Tetrahedral-octahedral sheets
Clay minerals consist of alternating tetrahedral and octahedral sheets forming layered structures, whereas silicate minerals primarily feature isolated or polymerized tetrahedral sheets without extensive octahedral layer integration.
Cation exchange capacity (CEC)
Clay minerals exhibit higher cation exchange capacity (CEC) than most silicate minerals due to their fine particle size and layered structure, which enhances their surface area and ability to retain and exchange cations.
Layered silicate structure
Clay minerals are a subset of silicate minerals characterized by their fine-grained, layered silicate structures composed of tetrahedral and octahedral sheets that influence their physical and chemical properties.
Kaolinite-smectite group
Kaolinite-smectite group minerals, crucial members of clay minerals, differ from broader silicate minerals due to their layered phyllosilicate structures, cation exchange capacity, and significant roles in soil fertility and industrial applications.
Isomorphous substitution
Isomorphous substitution in clay minerals involves the replacement of silicon or aluminum ions by other cations within the silicate structure, significantly influencing their cation exchange capacity and surface charge, whereas silicate minerals generally exhibit less extensive isomorphic substitution affecting their chemical properties.
Weathering products
Clay minerals are fine-grained weathering products primarily formed from the chemical alteration of silicate minerals through hydrolysis and leaching processes.
Swelling potential
Clay minerals exhibit high swelling potential due to their layered silicate structure and water absorption capacity, whereas most silicate minerals show minimal or no swelling.
Interlayer spacing
Clay minerals exhibit variable interlayer spacing due to water and cation exchange, whereas silicate minerals typically have fixed, rigid interlayer spacing determined by their crystal structure.
Aluminosilicate framework
Clay minerals feature a layered aluminosilicate framework formed by tetrahedral silica and octahedral alumina sheets, whereas silicate minerals encompass a broader group with diverse aluminosilicate structures including frameworks, chains, and sheets.
Clay minerals vs Silicate minerals Infographic
 njnir.com
njnir.com