3D bioprinting enables the precise fabrication of complex tissues by depositing bioinks layer by layer, facilitating cell viability and spatial organization that closely mimics natural tissue architecture. Electrospinning produces nanofibrous scaffolds with high surface area-to-volume ratios, promoting cell adhesion and nutrient exchange essential for tissue regeneration. Combining 3D bioprinting with electrospinning enhances scaffold functionalization, optimizing mechanical properties and biological performance for advanced biomedical applications.
Table of Comparison
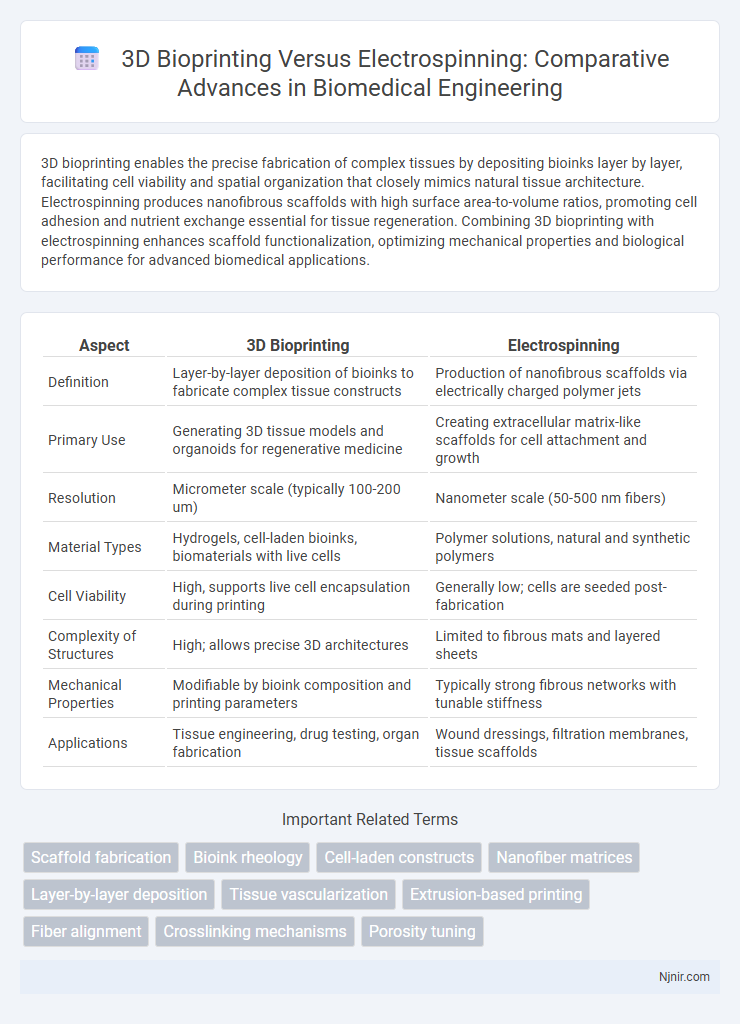
| Aspect | 3D Bioprinting | Electrospinning |
|---|---|---|
| Definition | Layer-by-layer deposition of bioinks to fabricate complex tissue constructs | Production of nanofibrous scaffolds via electrically charged polymer jets |
| Primary Use | Generating 3D tissue models and organoids for regenerative medicine | Creating extracellular matrix-like scaffolds for cell attachment and growth |
| Resolution | Micrometer scale (typically 100-200 um) | Nanometer scale (50-500 nm fibers) |
| Material Types | Hydrogels, cell-laden bioinks, biomaterials with live cells | Polymer solutions, natural and synthetic polymers |
| Cell Viability | High, supports live cell encapsulation during printing | Generally low; cells are seeded post-fabrication |
| Complexity of Structures | High; allows precise 3D architectures | Limited to fibrous mats and layered sheets |
| Mechanical Properties | Modifiable by bioink composition and printing parameters | Typically strong fibrous networks with tunable stiffness |
| Applications | Tissue engineering, drug testing, organ fabrication | Wound dressings, filtration membranes, tissue scaffolds |
Introduction to 3D Bioprinting and Electrospinning
3D bioprinting utilizes layer-by-layer deposition of bioinks composed of living cells and biomaterials to fabricate complex tissue constructs with precise spatial control, enabling customized tissue engineering applications. Electrospinning produces ultra-fine fibrous scaffolds through the application of a high-voltage electric field to a polymer solution, creating nanofibers that mimic the extracellular matrix structure critical for cell adhesion and proliferation. Both techniques are pivotal in regenerative medicine, with 3D bioprinting offering architectural precision and electrospinning providing biomimetic microenvironments ideal for tissue regeneration.
Core Principles of 3D Bioprinting
3D bioprinting relies on precise layer-by-layer deposition of bioinks containing living cells, biomaterials, and growth factors to fabricate complex tissue constructs that mimic natural architectures. This technique employs computer-aided design (CAD) and extrusion, inkjet, or laser-assisted printing modalities to achieve high-resolution spatial control and cell viability. Core principles emphasize maintaining biocompatibility, structural integrity, and functional viability throughout the printing process, distinguishing it from electrospinning, which produces fibrous scaffolds via the application of electrostatic forces without direct cell incorporation.
Fundamentals of Electrospinning Technology
Electrospinning technology utilizes electrostatic forces to produce fine polymer nanofibers, creating scaffolds that mimic the extracellular matrix essential for tissue engineering. This technique involves applying a high voltage to a polymer solution, which generates a charged jet that elongates and solidifies into continuous fibers with diameters ranging from nanometers to micrometers. Compared to 3D bioprinting, electrospinning offers superior control over fiber morphology and porosity, making it ideal for fabricating biomimetic structures with enhanced mechanical properties and cellular interactions.
Material Selection and Bioink Compatibility
Material selection in 3D bioprinting emphasizes hydrogels and bioinks composed of natural polymers like alginate, gelatin, and collagen, ensuring cell viability and precise deposition. In contrast, electrospinning predominantly uses synthetic polymers such as polycaprolactone (PCL) and polylactic acid (PLA), creating nanofibrous scaffolds that mimic extracellular matrix architecture but offer limited direct cell encapsulation. Bioink compatibility in 3D bioprinting hinges on rheological properties and crosslinking mechanisms that support live cell encapsulation, whereas electrospinning necessitates polymer solutions compatible with high-voltage fields, often constraining bioactive material integration.
Structural Complexity and Scaffold Architecture
3D bioprinting enables precise fabrication of complex, heterogeneous scaffolds with controlled spatial distribution of multiple cell types and biomaterials, closely mimicking native tissue architecture. Electrospinning produces nanofibrous scaffolds with high surface area and porosity but typically lacks the spatial control needed for intricate, multi-layered structures. The ability of 3D bioprinting to customize scaffold geometry at micro and macro scales offers significant advantages over electrospinning in replicating the hierarchical organization essential for functional tissue engineering.
Cellular Viability and Tissue Integration
3D bioprinting enables precise placement of living cells within bioinks, enhancing cellular viability through controlled microenvironments and nutrient diffusion. Electrospinning creates nanofibrous scaffolds that mimic the extracellular matrix, supporting tissue integration but often requires post-processing steps to seed cells effectively. Both methods offer distinct advantages, with 3D bioprinting favoring higher initial cell viability and electrospinning promoting structural support critical for long-term tissue regeneration.
Mechanical Properties and Functional Performance
3D bioprinting enables precise fabrication of complex tissue structures with customizable mechanical properties by layering biomaterials and cells, resulting in constructs that closely mimic native tissue stiffness and elasticity. Electrospinning produces nanofibrous scaffolds with high surface area and porosity, offering excellent mechanical strength and flexibility suitable for tissue engineering but with less control over cell placement and structural complexity. Functional performance in 3D bioprinting supports enhanced cell viability and biomimicry, while electrospun scaffolds excel in promoting cell adhesion and nutrient diffusion due to their fibrous architecture.
Clinical Applications and Biomedical Uses
3D bioprinting enables precise fabrication of complex tissue structures for regenerative medicine, organ transplantation, and wound healing, offering customizable patient-specific solutions. Electrospinning produces nanofibrous scaffolds that mimic natural extracellular matrices, enhancing cell attachment and proliferation in tissue engineering and drug delivery systems. Both techniques play pivotal roles in clinical applications, with 3D bioprinting advancing organ-on-a-chip models and electrospinning improving implant integration and controlled therapeutic release.
Current Limitations and Technological Challenges
3D bioprinting faces limitations in achieving vascularization and maintaining cell viability during longer fabrication processes, while electrospinning struggles with controlling fiber alignment and scalability for uniform tissue constructs. Both technologies encounter challenges in biomaterial compatibility, with 3D bioprinting requiring bioinks that balance mechanical strength and biocompatibility, whereas electrospinning demands polymers suitable for nanoscale fiber formation without cytotoxicity. Integration of multi-material printing and precise microarchitecture control remains a significant hurdle for advancing both methods in clinical tissue engineering applications.
Future Perspectives in Tissue Engineering
3D bioprinting and electrospinning are revolutionizing future tissue engineering by enabling precise fabrication of complex, functional tissues with tailored microenvironments. Advances in 3D bioprinting allow the integration of multiple cell types and biomaterials in spatially controlled architectures, enhancing tissue maturation and vascularization. Electrospinning's ability to create nanofibrous scaffolds closely mimics extracellular matrix structures, promoting cell adhesion and differentiation, with promising applications in regenerative medicine and personalized implants.
Scaffold fabrication
3D bioprinting enables precise scaffold fabrication with customizable geometry and cell placement, while electrospinning produces nanoscale fibrous scaffolds mimicking extracellular matrix with high surface area and porosity.
Bioink rheology
Bioink rheology in 3D bioprinting requires precise viscosity and shear-thinning properties for accurate extrusion, whereas electrospinning demands lower viscosity solutions with optimal viscoelasticity to form continuous nanofibers.
Cell-laden constructs
3D bioprinting enables precise fabrication of cell-laden constructs with high spatial control and viability, whereas electrospinning primarily produces acellular nanofibrous scaffolds with limited cell incorporation.
Nanofiber matrices
Nanofiber matrices produced by electrospinning offer higher surface area and porosity for cell attachment and nutrient diffusion compared to 3D bioprinting, which enables precise spatial control of multimaterial constructs in tissue engineering.
Layer-by-layer deposition
3D bioprinting achieves precise layer-by-layer deposition of bioinks for complex tissue structures, while electrospinning fabricates nanofibrous scaffolds through continuous fiber layering without direct cell placement control.
Tissue vascularization
3D bioprinting enables precise spatial placement of vascular cells and biomaterials to create complex, perfusable vascular networks, whereas electrospinning produces nanofibrous scaffolds that mimic extracellular matrix architecture but require additional techniques to support vascularization.
Extrusion-based printing
Extrusion-based 3D bioprinting offers precise layer-by-layer deposition of cell-laden bioinks enabling complex tissue fabrication, whereas electrospinning primarily produces nanoscale fiber scaffolds without direct cell placement, making extrusion bioprinting more suitable for constructing living tissue constructs.
Fiber alignment
3D bioprinting enables precise fiber alignment through controlled deposition patterns, whereas electrospinning offers nanoscale fiber alignment influenced by electric field parameters and collector design.
Crosslinking mechanisms
3D bioprinting utilizes photo- or enzymatic crosslinking to stabilize bioinks layer-by-layer, whereas electrospinning relies on physical or chemical crosslinking post-fiber formation to enhance scaffold mechanical properties and biocompatibility.
Porosity tuning
3D bioprinting enables precise porosity tuning through controlled layer-by-layer deposition, while electrospinning offers adjustable nanofiber porosity by manipulating fiber diameter and deposition parameters.
3D Bioprinting vs Electrospinning Infographic
 njnir.com
njnir.com