Bioprinting enables precise layer-by-layer deposition of living cells and biomaterials to create complex, functional tissue structures, enhancing regenerative medicine applications. Electrospinning produces nanofibrous scaffolds that mimic the extracellular matrix, promoting cell adhesion and proliferation in tissue engineering. Combining both techniques offers synergistic benefits, blending architectural control with nanoscale structural cues for advanced biomedical constructs.
Table of Comparison
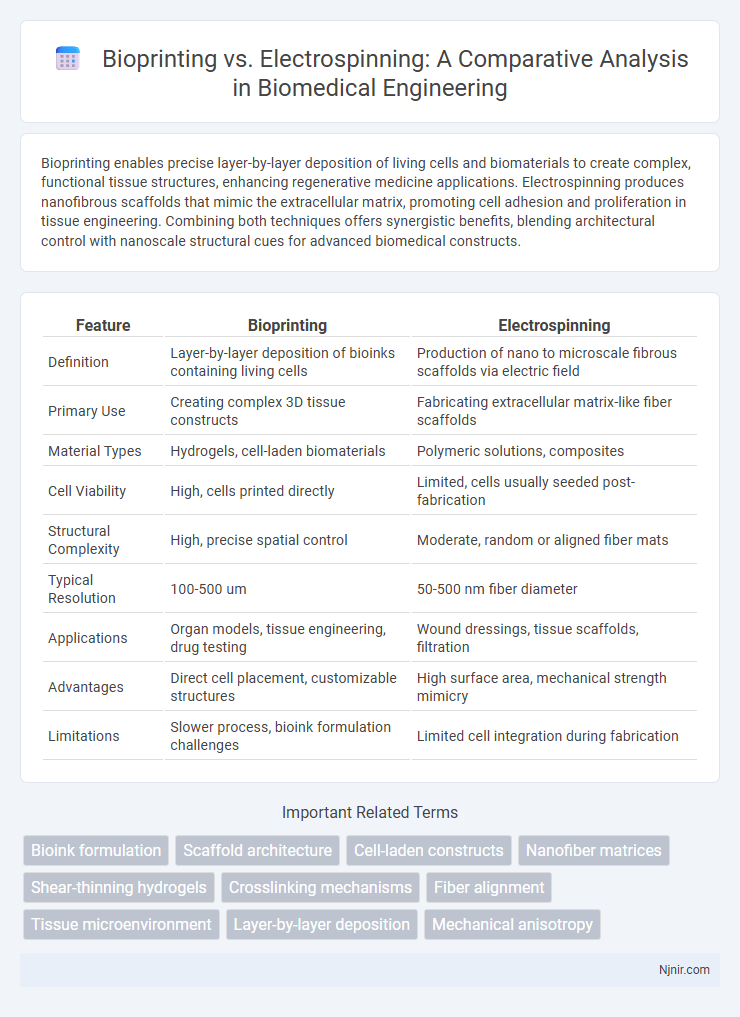
| Feature | Bioprinting | Electrospinning |
|---|---|---|
| Definition | Layer-by-layer deposition of bioinks containing living cells | Production of nano to microscale fibrous scaffolds via electric field |
| Primary Use | Creating complex 3D tissue constructs | Fabricating extracellular matrix-like fiber scaffolds |
| Material Types | Hydrogels, cell-laden biomaterials | Polymeric solutions, composites |
| Cell Viability | High, cells printed directly | Limited, cells usually seeded post-fabrication |
| Structural Complexity | High, precise spatial control | Moderate, random or aligned fiber mats |
| Typical Resolution | 100-500 um | 50-500 nm fiber diameter |
| Applications | Organ models, tissue engineering, drug testing | Wound dressings, tissue scaffolds, filtration |
| Advantages | Direct cell placement, customizable structures | High surface area, mechanical strength mimicry |
| Limitations | Slower process, bioink formulation challenges | Limited cell integration during fabrication |
Introduction to Bioprinting and Electrospinning
Bioprinting is an advanced 3D printing technique that fabricates biological structures by precisely depositing bioinks containing living cells and biomaterials. Electrospinning is a versatile method producing nanofibrous scaffolds through electrostatic forces that draw polymer solutions into fine fibers for tissue engineering applications. Both technologies enable the creation of customized tissue constructs, with bioprinting focusing on cellular placement and electrospinning emphasizing extracellular matrix mimicry.
Principles and Mechanisms of Bioprinting
Bioprinting utilizes layer-by-layer deposition of bioinks containing living cells and biomaterials to create precise three-dimensional tissue constructs, relying on techniques such as inkjet, extrusion, and laser-assisted printing. It operates through digital modeling and controlled spatial placement, ensuring cell viability and structural fidelity during the fabrication process. This contrasts with electrospinning, which produces nanofibrous scaffolds by applying a high-voltage electric field to a polymer solution, rather than assembling cell-laden constructs.
Fundamentals of Electrospinning Technology
Electrospinning technology utilizes electrostatic forces to produce ultrafine fibers from polymer solutions or melts, forming nanofibrous mats with high surface area-to-volume ratios essential for tissue engineering and drug delivery. Unlike bioprinting, which deposits bioinks layer-by-layer to create structured 3D constructs, electrospinning continuously generates fibers through a charged jet propelled toward a grounded collector, allowing control over fiber diameter and morphology. Fundamental parameters such as voltage, solution viscosity, flow rate, and collector distance critically influence fiber formation, making electrospinning a versatile technique for fabricating biomimetic scaffolds in regenerative medicine.
Material Compatibility in Bioprinting and Electrospinning
Material compatibility in bioprinting centers on hydrogels and bioinks, which must support cell viability and provide appropriate mechanical properties for tissue formation. Electrospinning primarily uses polymers such as polycaprolactone (PCL) and polylactic acid (PLA), focusing on creating fibrous scaffolds with high surface area and suitable porosity for cell attachment. Both techniques require biocompatible materials, but bioprinting demands precise rheological properties for extrusion or inkjet deposition, while electrospinning relies on polymer solution conductivity and viscosity for fiber formation.
Resolution and Structural Complexity Comparison
Bioprinting achieves high resolution typically around 100 microns, enabling precise deposition of cells and biomaterials to create complex, multicellular structures with spatial control. Electrospinning produces nanofiber scaffolds with fiber diameters ranging from 50 to 500 nanometers, offering superior nanoscale resolution but limited control over three-dimensional architecture. While bioprinting excels in fabricating anatomically accurate, heterogeneous tissues, electrospinning provides high surface area and porosity ideal for extracellular matrix mimicry yet lacks the structural complexity achievable through layer-by-layer bioprinting.
Applications in Tissue Engineering
Bioprinting enables precise placement of cells and biomaterials to create complex, functional tissue constructs suitable for organ regeneration and wound healing. Electrospinning produces nanofibrous scaffolds that mimic the extracellular matrix, promoting cell attachment and proliferation critical for bone, cartilage, and skin tissue engineering. Both technologies complement each other by combining bioprinting's architectural control with electrospinning's biomimetic fiber structures to enhance tissue regeneration outcomes.
Cell Viability and Integration Outcomes
Bioprinting offers precise spatial control of cell placement, enhancing cell viability and promoting better tissue integration by mimicking natural tissue architecture. Electrospinning creates nanofibrous scaffolds with high surface area, supporting cell attachment but often presenting challenges for uniform cell distribution and survival during scaffold fabrication. Studies show bioprinted constructs typically achieve higher cell viability rates above 80%, while electrospun scaffolds may require post-fabrication cell seeding to improve integration outcomes.
Scalability and Manufacturing Considerations
Bioprinting offers precise control over cell placement and complex tissue structures, making it suitable for personalized medicine but faces challenges in scalability due to slow layer-by-layer deposition and high costs. Electrospinning enables high-throughput production of nanofibrous scaffolds with consistent fiber morphology, providing a scalable and cost-effective approach for mass manufacturing of tissue engineering scaffolds. Manufacturing considerations favor electrospinning for bulk scaffold production, while bioprinting excels in customized, smaller-scale applications requiring intricate biological architecture.
Current Challenges and Limitations
Bioprinting faces challenges including limited resolution, slow fabrication speed, and maintaining cell viability during the printing process. Electrospinning struggles with controlling fiber alignment, scalability for industrial production, and difficulty incorporating living cells directly into the fibers. Both techniques require advancements in biomaterial development and process optimization to overcome these limitations for effective tissue engineering applications.
Future Prospects in Biomedical Engineering
Bioprinting offers precise, layer-by-layer construction of complex tissue structures with potential for personalized medicine and organ regeneration, while electrospinning excels in creating nanofibrous scaffolds that mimic extracellular matrix for enhanced cell growth and drug delivery. Advances in biomaterials and combined bioprinting-electrospinning platforms are expected to revolutionize tissue engineering by improving scaffold integration and vascularization. Future biomedical engineering trends emphasize hybrid technologies enabling functional tissue constructs with tailored mechanical and biological properties to address organ shortage and chronic disease treatment.
Bioink formulation
Bioink formulation in bioprinting involves precise customization of cell-laden hydrogels for tissue engineering, whereas electrospinning bioink focuses on creating nanofibrous scaffolds with polymer solutions to enhance cell adhesion and structural integrity.
Scaffold architecture
Bioprinting enables precise, customizable scaffold architecture with controlled pore size and geometry, while electrospinning produces nanofibrous scaffolds with high surface area but limited structural complexity.
Cell-laden constructs
Bioprinting enables precise placement of cell-laden bioinks for complex tissue architectures, while electrospinning produces fibrous scaffolds with enhanced mechanical properties but limited direct cell incorporation.
Nanofiber matrices
Nanofiber matrices produced by electrospinning offer superior structural mimicry and cell adhesion properties compared to bioprinting, which excels in complex tissue architecture but lacks the nanoscale fiber precision critical for regenerative medicine applications.
Shear-thinning hydrogels
Shear-thinning hydrogels enable precise bioprinting by reducing shear stress during extrusion, whereas electrospinning primarily produces nanofibrous scaffolds without utilizing shear-thinning properties.
Crosslinking mechanisms
Bioprinting employs photo- or ionic crosslinking to stabilize hydrogel-based bioinks, while electrospinning relies on chemical or physical crosslinking to enhance fiber integrity and mechanical properties.
Fiber alignment
Bioprinting offers precise control over fiber alignment through programmable deposition patterns, whereas electrospinning achieves fiber alignment primarily via collector design and electric field manipulation.
Tissue microenvironment
Bioprinting creates structured 3D tissue microenvironments with precise cell placement, while electrospinning produces fibrous scaffolds that mimic extracellular matrix architecture essential for cell adhesion and proliferation.
Layer-by-layer deposition
Bioprinting precisely deposits living cells layer-by-layer to create complex tissues, while electrospinning forms nanoscale fiber layers through high-voltage electric fields for scaffold fabrication.
Mechanical anisotropy
Bioprinting enables controlled architectural design but often lacks the inherent mechanical anisotropy achieved by electrospinning, which produces aligned fibrous structures mimicking native tissue mechanics.
Bioprinting vs Electrospinning Infographic
 njnir.com
njnir.com