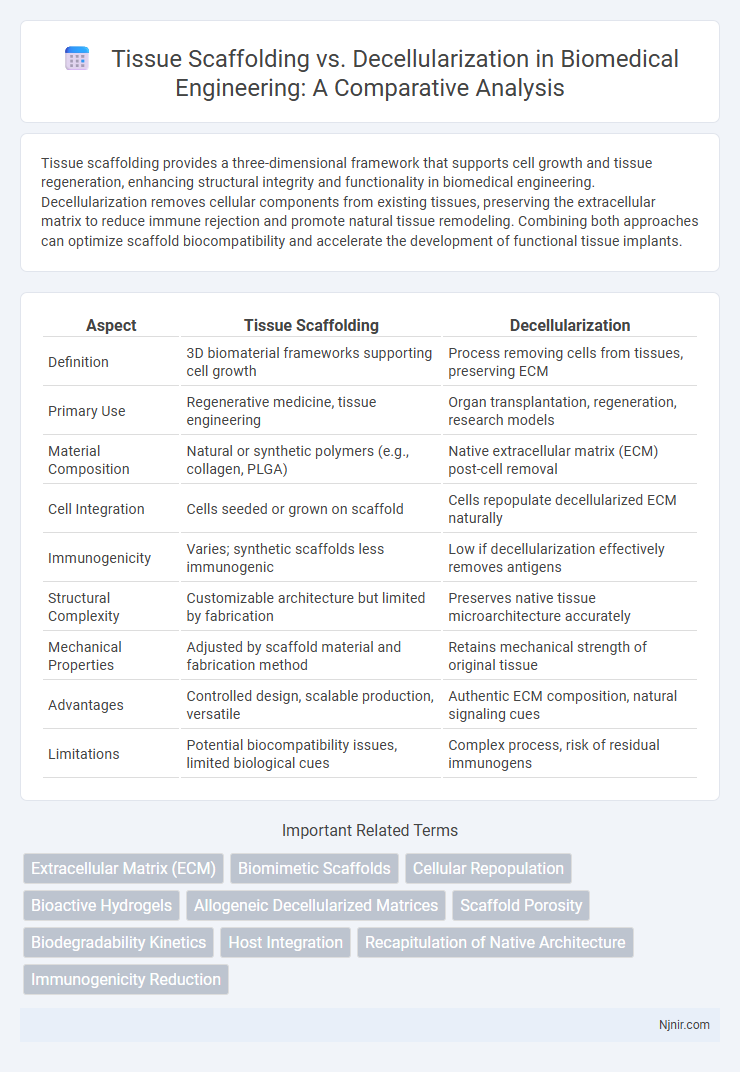
Tissue scaffolding provides a three-dimensional framework that supports cell growth and tissue regeneration, enhancing structural integrity and functionality in biomedical engineering. Decellularization removes cellular components from existing tissues, preserving the extracellular matrix to reduce immune rejection and promote natural tissue remodeling. Combining both approaches can optimize scaffold biocompatibility and accelerate the development of functional tissue implants.
Table of Comparison
| Aspect | Tissue Scaffolding | Decellularization |
|---|---|---|
| Definition | 3D biomaterial frameworks supporting cell growth | Process removing cells from tissues, preserving ECM |
| Primary Use | Regenerative medicine, tissue engineering | Organ transplantation, regeneration, research models |
| Material Composition | Natural or synthetic polymers (e.g., collagen, PLGA) | Native extracellular matrix (ECM) post-cell removal |
| Cell Integration | Cells seeded or grown on scaffold | Cells repopulate decellularized ECM naturally |
| Immunogenicity | Varies; synthetic scaffolds less immunogenic | Low if decellularization effectively removes antigens |
| Structural Complexity | Customizable architecture but limited by fabrication | Preserves native tissue microarchitecture accurately |
| Mechanical Properties | Adjusted by scaffold material and fabrication method | Retains mechanical strength of original tissue |
| Advantages | Controlled design, scalable production, versatile | Authentic ECM composition, natural signaling cues |
| Limitations | Potential biocompatibility issues, limited biological cues | Complex process, risk of residual immunogens |
Introduction to Tissue Engineering Approaches
Tissue scaffolding involves creating biomaterial frameworks that support cell attachment, growth, and differentiation, enabling the regeneration of damaged tissues in tissue engineering. Decellularization removes cellular components from donor tissues or organs, preserving the extracellular matrix structure to serve as a natural scaffold for cell repopulation. Both approaches aim to restore tissue function by combining biological compatibility with structural and mechanical cues essential for tissue regeneration.
Fundamentals of Tissue Scaffolding
Tissue scaffolding involves creating a three-dimensional structure composed of biocompatible materials that support cell attachment, proliferation, and differentiation, essential for regenerating damaged tissues. Unlike decellularization, which relies on removing cells from donor tissues to retain native extracellular matrix architecture, tissue scaffolding uses synthetic or natural polymers tailored for specific mechanical and biological properties. The fundamental aim is to mimic the extracellular matrix's physical and biochemical cues to promote tissue integration and functional restoration.
Principles of Decellularization Techniques
Decellularization techniques rely on removing cellular components from tissues while preserving the extracellular matrix (ECM) to maintain structural and biochemical cues essential for tissue regeneration. Common methods include chemical detergents like SDS and Triton X-100, enzymatic treatments using nucleases, and physical approaches such as freeze-thaw cycles or agitation to disrupt cell membranes. Effective decellularization balances cell removal with ECM integrity, enhancing biocompatibility and reducing immune response in tissue engineering applications.
Material Sources for Scaffolds
Tissue scaffolding primarily uses synthetic polymers like polylactic acid (PLA), polyglycolic acid (PGA), and natural biomaterials such as collagen and chitosan, offering controlled porosity and mechanical properties. Decellularization relies on native extracellular matrix (ECM) obtained from donor tissues or organs, preserving the natural biochemical cues and structural integrity critical for cell attachment and differentiation. Material sources for scaffolds in decellularization include animal-derived organs like porcine heart valves and human cadaveric tissues, providing a biologically relevant scaffold environment.
Structural and Mechanical Properties Comparison
Tissue scaffolding techniques often provide customizable architecture and enhanced mechanical strength, closely mimicking native extracellular matrix properties. Decellularization preserves the native tissue's complex microarchitecture and biochemical cues, maintaining intrinsic mechanical integrity, although it may exhibit variability due to donor tissue differences. Structural fidelity in decellularized scaffolds supports natural load-bearing capacity, while engineered scaffolds allow tunable stiffness and porosity tailored to specific regenerative applications.
Biocompatibility and Immune Response
Tissue scaffolding offers customizable support structures that promote cell attachment and growth, enhancing biocompatibility by mimicking native extracellular matrix properties. Decellularization involves removing cellular components from donor tissues to minimize immune response while preserving the natural architecture and biochemical cues essential for regeneration. Both methods aim to reduce rejection risks, but decellularized scaffolds typically elicit lower immunogenicity due to the reduced presence of antigenic cellular material.
Cellular Integration and Regeneration
Tissue scaffolding provides a three-dimensional framework that promotes cellular integration by facilitating cell attachment, proliferation, and differentiation essential for tissue regeneration. Decellularization preserves the native extracellular matrix architecture and bioactive cues, enhancing cellular infiltration and vascularization while minimizing immune rejection. Both approaches support regeneration, but decellularized scaffolds offer superior biochemical signals for tissue-specific cellular integration and functional recovery.
Clinical Applications and Case Studies
Tissue scaffolding provides a customizable matrix for cell growth in regenerative medicine, offering precise control over scaffold architecture and composition to enhance tissue integration in clinical applications such as bone and cartilage repair. Decellularization preserves native extracellular matrix structures, reducing immunogenicity and improving functional outcomes in organ transplantation and cardiovascular grafts, as demonstrated by successful case studies in heart valve and trachea replacement. Comparative clinical evidence highlights that while tissue scaffolding excels in tailored tissue engineering, decellularization remains superior for whole-organ regeneration due to its preservation of complex tissue microenvironments.
Challenges and Limitations
Tissue scaffolding faces challenges such as limited biocompatibility, immune rejection, and insufficient vascularization, which hinder tissue integration and long-term function. Decellularization struggles with incomplete removal of cellular components, leading to potential immune responses, as well as structural damage to the extracellular matrix that compromises mechanical strength. Both methods require advancements in biomaterial design and processing techniques to overcome issues of scalability and reproducibility for clinical applications.
Future Directions in Tissue Regeneration
Tissue scaffolding techniques are advancing with biomaterials that improve cell adhesion, growth, and differentiation, enabling more precise control over tissue architecture for regenerative medicine. Decellularization methods focus on preserving extracellular matrix components to provide a natural scaffold that supports cellular repopulation and functional tissue integration. Future directions emphasize combining these approaches with bioprinting and stem cell technologies to enhance tissue regeneration outcomes and personalized therapeutic applications.
Extracellular Matrix (ECM)
Tissue scaffolding replicates structural and biochemical cues of the extracellular matrix (ECM) using synthetic or natural materials, while decellularization preserves the native ECM architecture and bioactive components by removing cellular elements from donor tissues.
Biomimetic Scaffolds
Biomimetic scaffolds in tissue engineering leverage tissue scaffolding and decellularization techniques to replicate the native extracellular matrix, enhancing cell adhesion, proliferation, and differentiation for improved tissue regeneration outcomes.
Cellular Repopulation
Decellularization preserves native extracellular matrix architecture, promoting more efficient cellular repopulation compared to synthetic tissue scaffolding.
Bioactive Hydrogels
Bioactive hydrogels in tissue scaffolding provide customizable, cell-friendly environments enhancing tissue regeneration, whereas decellularization preserves native extracellular matrix complexity for improved biocompatibility and structural integrity.
Allogeneic Decellularized Matrices
Allogeneic decellularized matrices offer superior biocompatibility and reduced immune rejection compared to synthetic tissue scaffolding by preserving native extracellular matrix architecture and bioactive molecules.
Scaffold Porosity
Tissue scaffolding offers controlled scaffold porosity crucial for cell migration and nutrient diffusion, whereas decellularization preserves native extracellular matrix porosity but may present variability impacting tissue regeneration.
Biodegradability Kinetics
Tissue scaffolding demonstrates customizable biodegradability kinetics through material composition and structure, whereas decellularization offers naturally derived scaffolds with intrinsic biodegradability rates influenced by native extracellular matrix components.
Host Integration
Host integration in tissue scaffolding relies on biocompatible synthetic or natural materials promoting cellular infiltration, whereas decellularization uses extracellular matrix scaffolds from donor tissues enhancing native tissue remodeling and immune tolerance.
Recapitulation of Native Architecture
Tissue scaffolding provides customizable frameworks but decellularization better preserves the native extracellular matrix and microarchitecture essential for functional tissue recapitulation.
Immunogenicity Reduction
Tissue scaffolding offers controlled immunogenicity reduction by providing biomimetic structures, whereas decellularization minimizes immune response by removing cellular components while preserving extracellular matrix integrity.
Tissue scaffolding vs Decellularization Infographic
 njnir.com
njnir.com