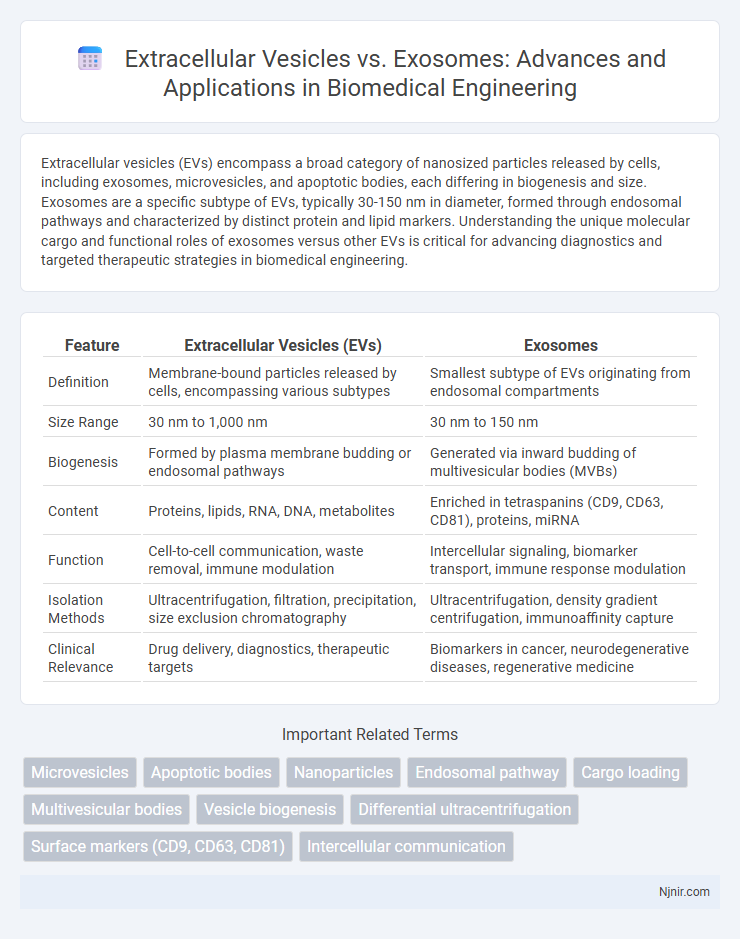
Extracellular vesicles (EVs) encompass a broad category of nanosized particles released by cells, including exosomes, microvesicles, and apoptotic bodies, each differing in biogenesis and size. Exosomes are a specific subtype of EVs, typically 30-150 nm in diameter, formed through endosomal pathways and characterized by distinct protein and lipid markers. Understanding the unique molecular cargo and functional roles of exosomes versus other EVs is critical for advancing diagnostics and targeted therapeutic strategies in biomedical engineering.
Table of Comparison
| Feature | Extracellular Vesicles (EVs) | Exosomes |
|---|---|---|
| Definition | Membrane-bound particles released by cells, encompassing various subtypes | Smallest subtype of EVs originating from endosomal compartments |
| Size Range | 30 nm to 1,000 nm | 30 nm to 150 nm |
| Biogenesis | Formed by plasma membrane budding or endosomal pathways | Generated via inward budding of multivesicular bodies (MVBs) |
| Content | Proteins, lipids, RNA, DNA, metabolites | Enriched in tetraspanins (CD9, CD63, CD81), proteins, miRNA |
| Function | Cell-to-cell communication, waste removal, immune modulation | Intercellular signaling, biomarker transport, immune response modulation |
| Isolation Methods | Ultracentrifugation, filtration, precipitation, size exclusion chromatography | Ultracentrifugation, density gradient centrifugation, immunoaffinity capture |
| Clinical Relevance | Drug delivery, diagnostics, therapeutic targets | Biomarkers in cancer, neurodegenerative diseases, regenerative medicine |
Introduction to Extracellular Vesicles and Exosomes
Extracellular vesicles (EVs) are heterogeneous, lipid bilayer-enclosed particles released by cells, including microvesicles, apoptotic bodies, and exosomes, each differing in size, biogenesis, and molecular content. Exosomes are a specific subtype of EVs, typically 30-150 nm in diameter, originating from the endosomal pathway through multivesicular body fusion with the plasma membrane. Their roles in intercellular communication, biomarker discovery, and therapeutic delivery highlight the importance of distinguishing between general extracellular vesicles and the more defined exosomes in biomedical research.
Defining Extracellular Vesicles: Types and Characteristics
Extracellular vesicles (EVs) are lipid bilayer-enclosed particles secreted by cells, encompassing diverse subtypes such as exosomes, microvesicles, and apoptotic bodies, each differing in size, biogenesis, and function. Exosomes, a well-characterized subset of EVs, typically measure 30-150 nm and originate from the endosomal pathway, playing critical roles in intercellular communication and molecular cargo transport. Understanding the distinct biogenetic pathways and molecular markers like tetraspanins (CD9, CD63, CD81) enables accurate differentiation between exosomes and other EV types, crucial for biomedical research and therapeutic applications.
Exosomes: Structure, Biogenesis, and Secretion
Exosomes are small extracellular vesicles, typically 30-150 nm in diameter, enclosed by a lipid bilayer enriched with tetraspanins such as CD63, CD81, and CD9, which serve as key structural markers. Their biogenesis involves the endosomal pathway, where intraluminal vesicles form within multivesicular bodies (MVBs) before fusing with the plasma membrane to release exosomes into the extracellular space. Exosome secretion is regulated by Rab GTPases, including Rab27a and Rab27b, and plays a crucial role in intercellular communication by transporting proteins, lipids, and RNA.
Key Differences between Extracellular Vesicles and Exosomes
Extracellular vesicles (EVs) encompass a broad category of membrane-bound particles released by cells, including exosomes, microvesicles, and apoptotic bodies, distinguished primarily by their biogenesis, size, and molecular content. Exosomes, a subtype of EVs, range from 30 to 150 nm and originate from the endosomal pathway as intraluminal vesicles within multivesicular bodies, later secreted into the extracellular space. Key differences include exosomes' specific protein markers such as CD9, CD63, and CD81, which are generally absent or less abundant in other EV types, and their distinct roles in intercellular communication and biomarker potential.
Isolation and Characterization Techniques
Isolation of extracellular vesicles (EVs) and exosomes primarily utilizes ultracentrifugation, size-exclusion chromatography, and immunoaffinity capture methods, each offering varying specificity and yield. Characterization techniques involve nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), and flow cytometry to assess size distribution, morphological features, and surface markers such as CD63, CD81, and CD9. High-resolution mass spectrometry and Western blotting further confirm molecular content, distinguishing exosomes as a subset of EVs based on their endosomal origin and protein profile.
Roles in Cell-to-Cell Communication
Extracellular vesicles (EVs) are membrane-bound particles released by cells, including exosomes, microvesicles, and apoptotic bodies, that facilitate cell-to-cell communication by transferring proteins, lipids, and nucleic acids. Exosomes, a subtype of EVs ranging from 30-150 nm, play a pivotal role in modulating recipient cell functions through selective cargo delivery, influencing processes such as immune response, tumor progression, and tissue regeneration. The distinct biogenesis pathways and cargo sorting mechanisms of exosomes enable precise intercellular signaling compared to the broader and more heterogeneous EV population.
Biomedical Applications: Diagnostics and Therapeutics
Extracellular vesicles (EVs), encompassing exosomes as a key subtype, exhibit distinct biomolecular cargo that enables precise diagnostic biomarker identification for diseases such as cancer and neurodegenerative disorders. Exosomes, typically 30-150 nm in size, demonstrate superior therapeutic delivery capabilities due to their ability to cross biological barriers and mediate targeted intercellular communication. Both EVs and exosomes are increasingly utilized in liquid biopsy platforms and regenerative medicine, highlighting their critical role in non-invasive diagnostics and personalized treatment strategies.
Challenges in Detection and Standardization
Extracellular vesicles (EVs) and exosomes present significant challenges in detection due to their overlapping size ranges (30-150 nm for exosomes and 30 nm to 1 um for EVs) and heterogeneous compositions, complicating isolation and characterization techniques such as ultracentrifugation and flow cytometry. Standardization difficulties arise from the lack of universally accepted markers distinguishing exosomes from other EV subtypes, leading to variability in experimental protocols and data interpretation across laboratories. Improving detection sensitivity and establishing consensus on isolation standards and biomarkers are crucial for advancing the reproducibility and clinical translation of EV research.
Clinical Relevance: Disease Biomarkers and Targeted Delivery
Extracellular vesicles (EVs) encompass various subtypes, including exosomes, which are nanosized vesicles crucial for intercellular communication and have emerged as valuable disease biomarkers due to their cargo of proteins, lipids, and nucleic acids reflecting pathological states. Exosomes, distinguished by their endosomal origin, exhibit high specificity and stability in biological fluids, making them particularly promising for non-invasive diagnostics in cancer, neurodegenerative disorders, and cardiovascular diseases. In targeted delivery, exosomes demonstrate superior biocompatibility and intrinsic homing capabilities, enhancing therapeutic efficacy and minimizing off-target effects compared to broader EV populations or synthetic nanocarriers.
Future Perspectives and Emerging Trends in Extracellular Vesicles Research
Emerging trends in extracellular vesicles research highlight the increasing differentiation between extracellular vesicles (EVs) and exosomes, emphasizing advancements in isolation techniques, such as microfluidics and high-resolution flow cytometry, to accurately characterize subpopulations and their cargo for diagnostic and therapeutic applications. Future perspectives focus on the integration of multi-omics approaches and artificial intelligence to unravel the complex biogenesis pathways and functional heterogeneity of EVs, enhancing their potential as precision medicine tools in oncology, neurodegenerative diseases, and regenerative therapies. Personalized EV-based drug delivery systems and scalable production methods are gaining momentum, driving translational research toward clinical-grade formulations and regulatory frameworks for widespread therapeutic use.
Microvesicles
Microvesicles, a subtype of extracellular vesicles ranging from 100 to 1000 nm, are shed directly from the plasma membrane, distinguishing them from smaller exosomes formed within endosomal compartments.
Apoptotic bodies
Apoptotic bodies, a type of extracellular vesicle larger than exosomes, are membrane-bound particles released during programmed cell death that carry cellular debris and signaling molecules essential for intercellular communication and immune response modulation.
Nanoparticles
Extracellular vesicles encompass a broad category of lipid-bound nanoparticles including exosomes, which are specifically small (30-150 nm) nanoparticles derived from endosomal pathways and crucial for intercellular communication and targeted drug delivery.
Endosomal pathway
Extracellular vesicles encompass various subtypes including exosomes, which specifically originate from the endosomal pathway through the inward budding of multivesicular bodies before their fusion with the plasma membrane.
Cargo loading
Extracellular vesicles encompass various subtypes including exosomes, which uniquely demonstrate selective cargo loading mechanisms involving sorting of proteins, lipids, and nucleic acids via endosomal pathways distinct from other vesicles.
Multivesicular bodies
Multivesicular bodies are key cellular structures that generate exosomes, a specific subtype of extracellular vesicles distinguished by their endosomal origin and unique biogenesis pathway.
Vesicle biogenesis
Extracellular vesicles encompass diverse vesicle types including exosomes, which specifically originate from the endosomal pathway through inward budding of multivesicular bodies, whereas other extracellular vesicles like microvesicles arise directly from plasma membrane outward budding during vesicle biogenesis.
Differential ultracentrifugation
Differential ultracentrifugation separates extracellular vesicles such as exosomes based on size and density, enabling isolation of exosomes typically at 100,000 x g after removing larger vesicles through sequential lower-speed spins.
Surface markers (CD9, CD63, CD81)
Extracellular vesicles and exosomes are differentiated by their surface markers, with exosomes typically enriched in tetraspanins CD9, CD63, and CD81, which serve as key identifiers for these nanosized vesicles involved in intercellular communication.
Intercellular communication
Extracellular vesicles, including exosomes, are key mediators of intercellular communication by transporting proteins, lipids, and nucleic acids that modulate cellular functions and signaling pathways.
Extracellular vesicles vs Exosomes Infographic
 njnir.com
njnir.com