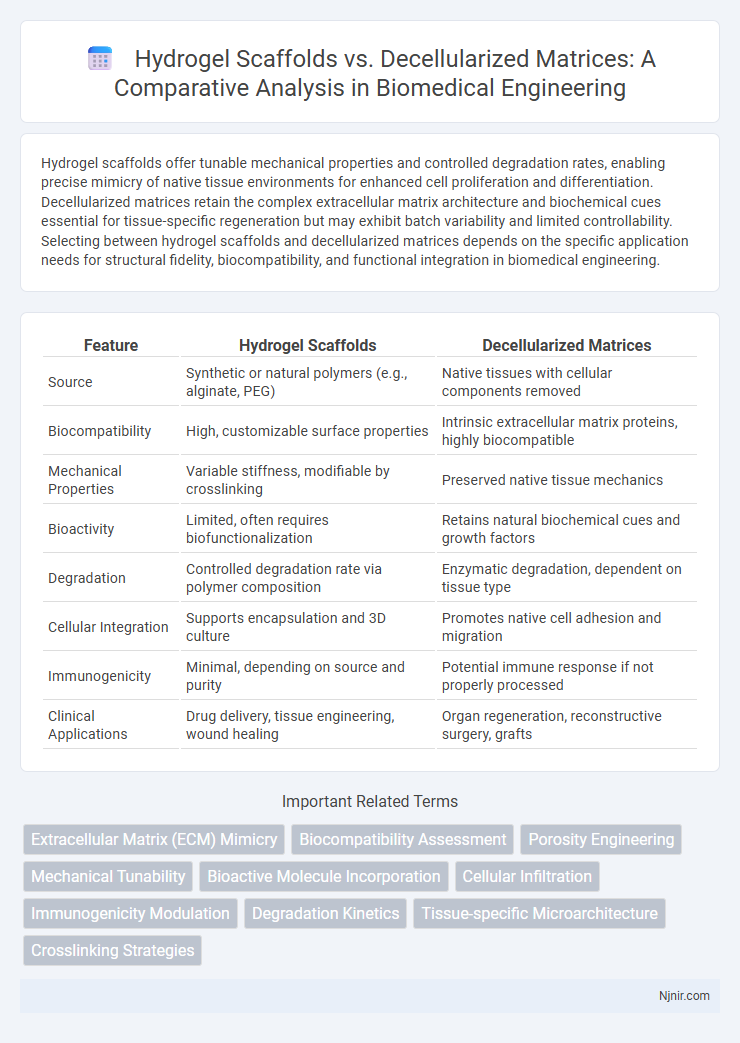
Hydrogel scaffolds offer tunable mechanical properties and controlled degradation rates, enabling precise mimicry of native tissue environments for enhanced cell proliferation and differentiation. Decellularized matrices retain the complex extracellular matrix architecture and biochemical cues essential for tissue-specific regeneration but may exhibit batch variability and limited controllability. Selecting between hydrogel scaffolds and decellularized matrices depends on the specific application needs for structural fidelity, biocompatibility, and functional integration in biomedical engineering.
Table of Comparison
| Feature | Hydrogel Scaffolds | Decellularized Matrices |
|---|---|---|
| Source | Synthetic or natural polymers (e.g., alginate, PEG) | Native tissues with cellular components removed |
| Biocompatibility | High, customizable surface properties | Intrinsic extracellular matrix proteins, highly biocompatible |
| Mechanical Properties | Variable stiffness, modifiable by crosslinking | Preserved native tissue mechanics |
| Bioactivity | Limited, often requires biofunctionalization | Retains natural biochemical cues and growth factors |
| Degradation | Controlled degradation rate via polymer composition | Enzymatic degradation, dependent on tissue type |
| Cellular Integration | Supports encapsulation and 3D culture | Promotes native cell adhesion and migration |
| Immunogenicity | Minimal, depending on source and purity | Potential immune response if not properly processed |
| Clinical Applications | Drug delivery, tissue engineering, wound healing | Organ regeneration, reconstructive surgery, grafts |
Introduction to Hydrogel Scaffolds and Decellularized Matrices
Hydrogel scaffolds are three-dimensional, hydrophilic polymer networks that mimic the extracellular matrix (ECM) by providing a hydrated environment conducive to cell growth and tissue regeneration. Decellularized matrices, derived from native tissues through the removal of cellular components, preserve the complex biochemical composition and structural integrity of the ECM, offering a natural scaffold for tissue engineering. Both materials serve as critical platforms in regenerative medicine, with hydrogel scaffolds offering tunable physical and chemical properties, while decellularized matrices provide inherent biological cues essential for cell adhesion and differentiation.
Structural Composition and Properties
Hydrogel scaffolds are composed of highly hydrated polymer networks that mimic the extracellular matrix's physical properties, offering tunable porosity and mechanical strength to support cell proliferation and differentiation. Decellularized matrices retain the native tissue architecture and biochemical cues by removing cellular components while preserving collagen, elastin, and glycosaminoglycans, which maintain natural tissue mechanics and promote cellular adhesion. The structural composition of hydrogel scaffolds allows for customizable elasticity and degradation rates, whereas decellularized matrices provide an inherently complex microenvironment with native vascular channels and extracellular matrix proteins that improve tissue integration.
Methods of Synthesis and Fabrication
Hydrogel scaffolds are synthesized through chemical or physical crosslinking of natural or synthetic polymers such as polyethylene glycol, alginate, or collagen, allowing precise control over porosity and mechanical properties via methods like photopolymerization, ionic crosslinking, or temperature-induced gelation. Decellularized matrices derive from native tissues or organs through physical, chemical, or enzymatic treatments that remove cellular components while preserving the extracellular matrix architecture and composition, with common protocols involving detergents, nucleases, and mechanical agitation. Fabrication of hydrogel scaffolds enables tunable structural features for specific tissue engineering applications, whereas decellularized matrices inherently maintain native biochemical cues and microstructure essential for recellularization and functional integration.
Biocompatibility and Immunogenicity
Hydrogel scaffolds exhibit high biocompatibility due to their tunable physicochemical properties and ability to mimic native extracellular matrix environments, reducing adverse immune responses. Decellularized matrices provide natural tissue architecture with preserved bioactive molecules, but their immunogenicity depends heavily on the effectiveness of the decellularization process in removing cellular antigens. Comparative studies show hydrogel scaffolds typically induce lower immune activation, while decellularized matrices may trigger variable immunogenicity linked to residual DNA and cellular debris.
Mechanical Strength and Stability
Hydrogel scaffolds typically exhibit lower mechanical strength and stability compared to decellularized matrices due to their highly hydrated polymer network, which limits load-bearing capacity and long-term durability. Decellularized matrices retain the native extracellular matrix's complex architecture and crosslinking, providing superior mechanical integrity and resistance to enzymatic degradation. Consequently, decellularized matrices are often preferred for applications requiring robust mechanical support and prolonged scaffold functionality.
Cellular Interactions and Integration
Hydrogel scaffolds provide a highly tunable three-dimensional environment that supports cellular adhesion, proliferation, and differentiation through customizable biochemical and mechanical cues, promoting enhanced cell-matrix interactions. Decellularized matrices retain native extracellular matrix architecture and composition, preserving essential biochemical signals and structural proteins that facilitate natural cell integration and tissue-specific cellular responses. Comparative studies show that decellularized matrices often yield superior cellular integration due to their inherent bioactivity, while hydrogel scaffolds offer versatility in modulating cell behavior for tailored tissue engineering applications.
Applications in Tissue Engineering
Hydrogel scaffolds provide a highly tunable environment with controlled porosity and mechanical properties, making them ideal for promoting cell proliferation and differentiation in tissue engineering applications such as cartilage and wound healing. Decellularized matrices retain native extracellular matrix components and three-dimensional architecture, offering superior bioactivity and immune compatibility for regenerating complex tissues like heart valves and liver. Both scaffold types play crucial roles, with hydrogels excelling in customizability and decellularized matrices in preserving native tissue microenvironment.
Advantages and Limitations
Hydrogel scaffolds offer tunable mechanical properties and high biocompatibility, enabling precise control over cell microenvironments, but often exhibit limited structural integrity and slower integration with host tissues. Decellularized matrices provide native extracellular matrix architecture and biochemical cues essential for tissue regeneration, yet carry risks of immunogenicity and inconsistent batch quality. Both scaffolding approaches require balancing biofunctionality with clinical applicability depending on tissue-specific repair goals.
Recent Advances and Innovations
Recent advances in hydrogel scaffolds emphasize tunable mechanical properties and bioactive molecule incorporation, enhancing cellular adhesion and proliferation for tissue engineering applications. Innovations in decellularized matrices involve refined decellularization techniques that preserve extracellular matrix (ECM) integrity and native biochemical cues, promoting improved recellularization and functional tissue regeneration. Emerging hybrid approaches combine hydrogel scaffolds with decellularized matrices, leveraging the advantages of both materials to optimize scaffold biocompatibility and regenerative potential.
Future Perspectives in Biomedical Engineering
Hydrogel scaffolds offer customizable mechanical properties and controlled bioactive factor delivery, enabling precise tissue regeneration and enhanced cell proliferation. Decellularized matrices provide native extracellular matrix architecture and biochemical cues, promoting improved cell differentiation and integration in tissue engineering applications. Future advancements in biomedical engineering may combine these approaches to create hybrid scaffolds that optimize cellular responses and accelerate functional tissue repair.
Extracellular Matrix (ECM) Mimicry
Hydrogel scaffolds offer tunable biochemical and mechanical properties that closely mimic the native extracellular matrix (ECM) environment, while decellularized matrices provide naturally preserved ECM components and architecture critical for cell adhesion and signaling.
Biocompatibility Assessment
Hydrogel scaffolds demonstrate superior biocompatibility with reduced cytotoxicity and enhanced cell viability compared to decellularized matrices, which may provoke immune responses due to residual antigens.
Porosity Engineering
Hydrogel scaffolds enable precise porosity engineering through tunable crosslinking and polymer concentration, enhancing cell infiltration and nutrient diffusion compared to the fixed, natural porosity of decellularized matrices.
Mechanical Tunability
Hydrogel scaffolds offer superior mechanical tunability through customizable crosslinking densities and polymer compositions, whereas decellularized matrices provide inherent but less adjustable mechanical properties derived from native tissue architecture.
Bioactive Molecule Incorporation
Hydrogel scaffolds enable precise bioactive molecule incorporation through controlled release systems, whereas decellularized matrices naturally retain native bioactive factors but offer limited customization for molecular delivery.
Cellular Infiltration
Hydrogel scaffolds generally enable higher cellular infiltration due to their tunable porosity and biochemical properties, whereas decellularized matrices offer native extracellular matrix architecture but often exhibit limited cell penetration.
Immunogenicity Modulation
Hydrogel scaffolds allow precise control over immunogenicity modulation by enabling customizable biochemical cues, whereas decellularized matrices inherently present native extracellular matrix components that can trigger variable immune responses depending on the efficacy of cell removal.
Degradation Kinetics
Hydrogel scaffolds offer tunable degradation kinetics adjustable through polymer composition and crosslinking density, whereas decellularized matrices exhibit variable, tissue-specific degradation rates influenced by native extracellular matrix enzymatic susceptibility.
Tissue-specific Microarchitecture
Hydrogel scaffolds offer tunable physical properties but often lack the complex, tissue-specific microarchitecture inherently preserved in decellularized matrices essential for directing cellular behavior and tissue regeneration.
Crosslinking Strategies
Crosslinking strategies in hydrogel scaffolds utilize chemical agents like glutaraldehyde and enzymatic methods such as transglutaminase to enhance mechanical stability, whereas decellularized matrices rely predominantly on physical crosslinking through freeze-thaw cycles and ultraviolet irradiation to preserve native extracellular matrix architecture.
hydrogel scaffolds vs decellularized matrices Infographic
 njnir.com
njnir.com