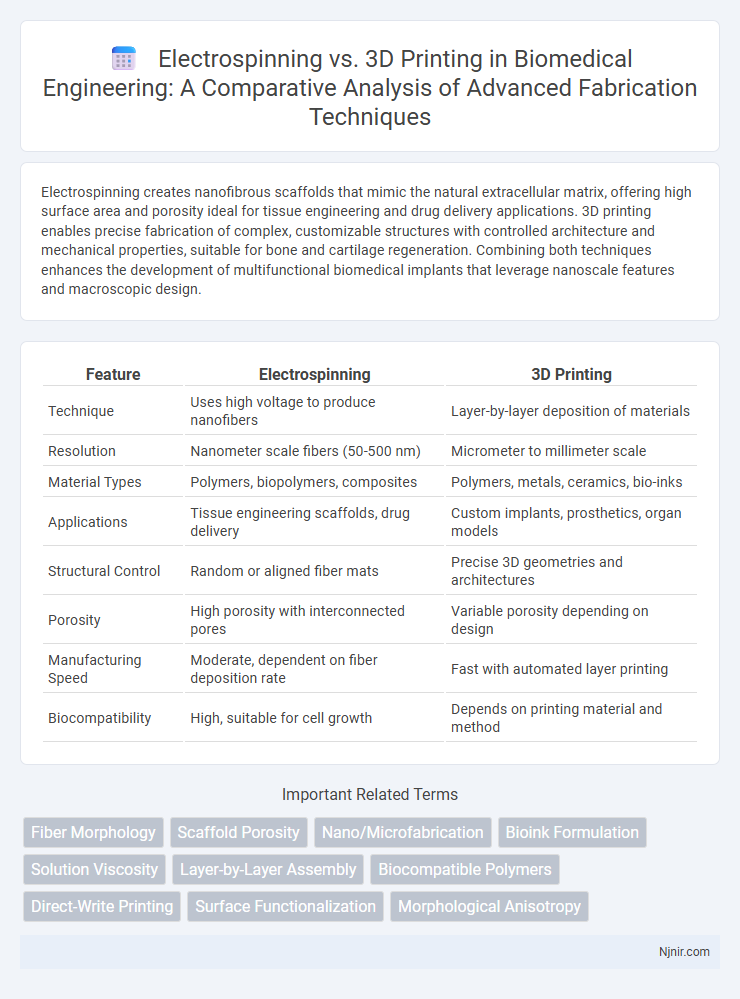
Electrospinning creates nanofibrous scaffolds that mimic the natural extracellular matrix, offering high surface area and porosity ideal for tissue engineering and drug delivery applications. 3D printing enables precise fabrication of complex, customizable structures with controlled architecture and mechanical properties, suitable for bone and cartilage regeneration. Combining both techniques enhances the development of multifunctional biomedical implants that leverage nanoscale features and macroscopic design.
Table of Comparison
| Feature | Electrospinning | 3D Printing |
|---|---|---|
| Technique | Uses high voltage to produce nanofibers | Layer-by-layer deposition of materials |
| Resolution | Nanometer scale fibers (50-500 nm) | Micrometer to millimeter scale |
| Material Types | Polymers, biopolymers, composites | Polymers, metals, ceramics, bio-inks |
| Applications | Tissue engineering scaffolds, drug delivery | Custom implants, prosthetics, organ models |
| Structural Control | Random or aligned fiber mats | Precise 3D geometries and architectures |
| Porosity | High porosity with interconnected pores | Variable porosity depending on design |
| Manufacturing Speed | Moderate, dependent on fiber deposition rate | Fast with automated layer printing |
| Biocompatibility | High, suitable for cell growth | Depends on printing material and method |
Introduction to Electrospinning and 3D Printing in Biomedical Engineering
Electrospinning creates nanofibrous scaffolds by using an electric field to draw charged polymer solutions into fine fibers, ideal for mimicking extracellular matrices in tissue engineering. 3D printing builds precise, customizable biomedical implants or devices layer-by-layer from digital designs, offering versatility in material choice and complex geometries. Both technologies are critical in advancing regenerative medicine by enabling tailored tissue scaffolds and patient-specific medical solutions.
Basic Principles: How Electrospinning and 3D Printing Work
Electrospinning utilizes an electric field to draw charged polymer fibers from a solution or melt, creating nanoscale mats with high surface area. In contrast, 3D printing builds objects layer-by-layer by extruding, sintering, or depositing materials such as plastics, metals, or ceramics based on digital models. Electrospinning excels in fabricating fine fibrous structures, while 3D printing offers precise control over complex geometries and macro-scale designs.
Material Compatibility: Polymers and Biomaterials
Electrospinning excels at processing a wide range of polymers and biomaterials, including natural polymers like collagen and synthetic polymers such as polycaprolactone (PCL), enabling the creation of nanofibrous scaffolds resembling extracellular matrices. In contrast, 3D printing accommodates diverse materials from thermoplastics like PLA and ABS to hydrogels and bioinks, offering precise control over macro-scale geometries but limited fibrous nanoscale structures. Material compatibility in electrospinning favors fine fiber formation for tissue engineering, while 3D printing supports structural complexity and multi-material integration for functional biomedical devices.
Structural Differences: Fiber Networks vs Layered Constructs
Electrospinning produces fibrous networks composed of nanometer-scale fibers creating highly porous, interconnected structures ideal for tissue engineering and filtration. In contrast, 3D printing builds layered constructs with precise geometries by depositing material in successive layers, enabling complex shapes and mechanical strength customization. The nanoscale fiber orientation in electrospinning differs fundamentally from the macroscale, layer-by-layer assembly characteristic of 3D printing.
Applications in Tissue Engineering and Regenerative Medicine
Electrospinning creates nanofibrous scaffolds that mimic the extracellular matrix, promoting cell adhesion and proliferation in tissue engineering applications such as skin regeneration and vascular grafts. 3D printing enables precise fabrication of complex, patient-specific tissue constructs with controlled pore architecture, enhancing regenerative medicine efforts for bone repair and organ regeneration. Combining both technologies offers synergistic advantages, improving scaffold functionality and tissue integration in advanced therapeutic strategies.
Customization and Design Capabilities
Electrospinning offers high customization at the nanoscale, enabling precise control over fiber morphology, diameter, and alignment for tailored tissue engineering scaffolds or filtration membranes. In contrast, 3D printing excels in designing complex macroscopic structures with intricate geometries and multi-material integration, supporting rapid prototyping and personalized medical implants. Both techniques provide unique design capabilities, with electrospinning favoring nanoscale customization and 3D printing enabling versatile, large-scale structural customization.
Mechanical Properties and Performance
Electrospinning produces nanofibrous scaffolds with high surface area and excellent tensile strength, ideal for applications requiring enhanced mechanical flexibility and durability. 3D printing offers superior control over macrostructure and mechanical properties such as stiffness and load-bearing capacity, enabling the creation of complex, customized geometries with high precision. While electrospun materials excel in fiber-level mechanical performance, 3D printed structures provide better overall mechanical integrity suited for load-bearing applications.
Scalability and Production Efficiency
Electrospinning offers high scalability for producing nanofibers with excellent control over fiber diameter and morphology, making it ideal for mass production in industries like tissue engineering and filtration. 3D printing excels in production efficiency for customized, complex geometries but faces scalability challenges due to slower build speeds and higher costs per unit. Manufacturing workflows benefit from combining electrospinning's rapid fiber generation with 3D printing's design flexibility to optimize overall output quality and volume.
Biocompatibility and Cell Interaction
Electrospinning generates nanofibrous scaffolds that closely mimic the extracellular matrix, enhancing cell adhesion, proliferation, and differentiation due to their high surface area and porosity. In contrast, 3D printing allows precise control over scaffold architecture and pore size but may face challenges in achieving the nanoscale features essential for optimal cell interaction. Biocompatibility of both techniques depends on the choice of biomaterials, with electrospun fibers often exhibiting superior integration in tissue engineering applications where nanoscale cues influence cellular responses.
Future Perspectives: Integrative Approaches and Innovations
Electrospinning and 3D printing technologies are converging to create hybrid fabrication methods that combine nanoscale fiber precision with complex 3D architectures, enhancing applications in tissue engineering and advanced manufacturing. Innovations in materials science enable the integration of electrospun nanofibers within 3D printed scaffolds, improving mechanical strength, cellular interaction, and functional performance. Future perspectives emphasize scalable, multi-material printing systems with real-time monitoring and AI-driven design, driving personalized medicine and smart device development.
Fiber Morphology
Electrospinning produces nanometer-scale fibers with high surface area and tunable porosity, whereas 3D printing creates microscale structures with defined geometries but limited control over fiber morphology.
Scaffold Porosity
Electrospinning produces scaffolds with high porosity and nanofiber networks ideal for cell attachment, while 3D printing creates scaffolds with controlled, customizable macroporosity tailored to specific tissue engineering applications.
Nano/Microfabrication
Electrospinning enables the fabrication of continuous nanofibers with high surface area for tissue engineering and filtration, while 3D printing excels in precise micro-scale geometries and complex scaffold designs for biomedical and microfabrication applications.
Bioink Formulation
Electrospinning offers precise nanoscale fiber fabrication ideal for creating biomimetic extracellular matrices, while 3D printing provides customizable, cell-laden bioink structures with controlled spatial architecture, making bioink formulation critical for balancing viscosity, cell viability, and mechanical properties in regenerative medicine applications.
Solution Viscosity
Solution viscosity critically influences electrospinning by determining fiber formation and morphology, whereas in 3D printing, it primarily affects extrusion flow and layer adhesion.
Layer-by-Layer Assembly
Electrospinning creates nanoscale fibrous mats through continuous fiber deposition, while 3D printing builds precise, macroscale objects via controlled layer-by-layer material extrusion.
Biocompatible Polymers
Electrospinning produces nanofibrous scaffolds with high surface area and porosity ideal for biocompatible polymers, while 3D printing enables precise control over complex geometries and mechanical properties suited for customized tissue engineering applications.
Direct-Write Printing
Direct-write printing in 3D printing enables precise layer-by-layer deposition for complex structures, contrasting with electrospinning's nanoscale fiber fabrication through electrically charged jets.
Surface Functionalization
Electrospinning offers superior surface functionalization through nanoscale fiber morphology and high surface area facilitating enhanced chemical modifications compared to 3D printing's layer-by-layer construction with limited surface complexity.
Morphological Anisotropy
Electrospinning produces nanofibrous mats with high morphological anisotropy due to aligned fiber deposition, whereas 3D printing typically yields isotropic or less anisotropic structures depending on print path and layer orientation.
Electrospinning vs 3D Printing Infographic
 njnir.com
njnir.com