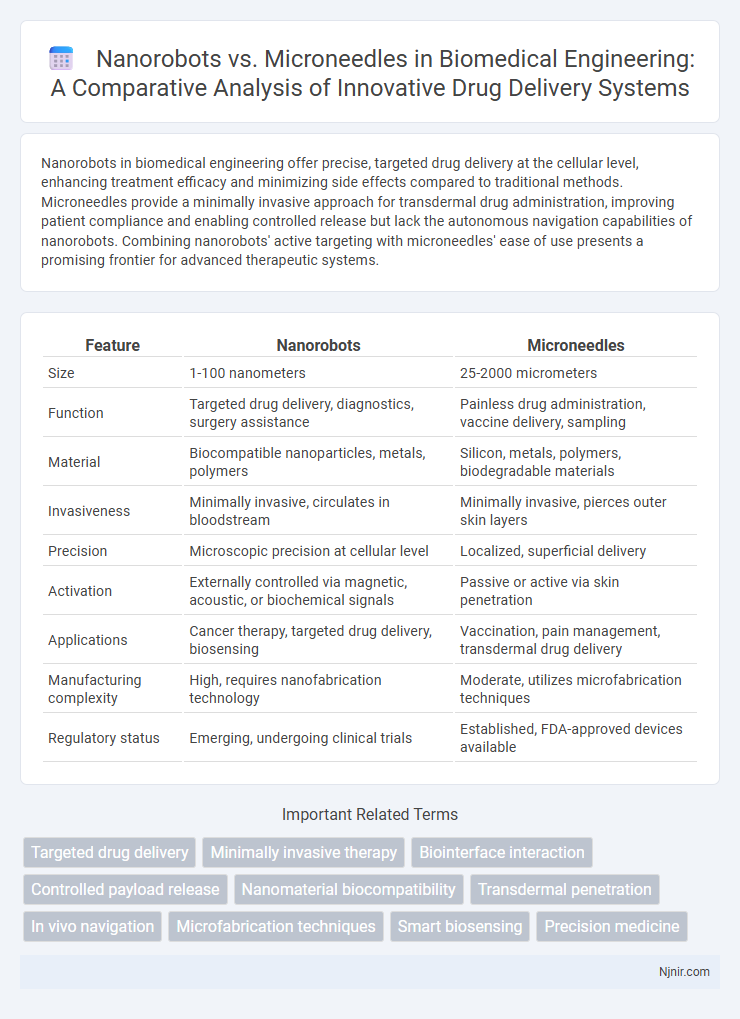
Nanorobots in biomedical engineering offer precise, targeted drug delivery at the cellular level, enhancing treatment efficacy and minimizing side effects compared to traditional methods. Microneedles provide a minimally invasive approach for transdermal drug administration, improving patient compliance and enabling controlled release but lack the autonomous navigation capabilities of nanorobots. Combining nanorobots' active targeting with microneedles' ease of use presents a promising frontier for advanced therapeutic systems.
Table of Comparison
| Feature | Nanorobots | Microneedles |
|---|---|---|
| Size | 1-100 nanometers | 25-2000 micrometers |
| Function | Targeted drug delivery, diagnostics, surgery assistance | Painless drug administration, vaccine delivery, sampling |
| Material | Biocompatible nanoparticles, metals, polymers | Silicon, metals, polymers, biodegradable materials |
| Invasiveness | Minimally invasive, circulates in bloodstream | Minimally invasive, pierces outer skin layers |
| Precision | Microscopic precision at cellular level | Localized, superficial delivery |
| Activation | Externally controlled via magnetic, acoustic, or biochemical signals | Passive or active via skin penetration |
| Applications | Cancer therapy, targeted drug delivery, biosensing | Vaccination, pain management, transdermal drug delivery |
| Manufacturing complexity | High, requires nanofabrication technology | Moderate, utilizes microfabrication techniques |
| Regulatory status | Emerging, undergoing clinical trials | Established, FDA-approved devices available |
Introduction to Nanorobots and Microneedles in Biomedical Engineering
Nanorobots are microscopic devices designed to perform precise tasks at the cellular or molecular level, revolutionizing targeted drug delivery and diagnostics in biomedical engineering. Microneedles are tiny, minimally invasive tools engineered to create microchannels in the skin for controlled drug administration and vaccine delivery. Both technologies enhance therapeutic efficiency by improving drug bioavailability and reducing patient discomfort through innovative biomedical applications.
Historical Evolution: From Microneedles to Nanorobots
Microneedles emerged in the 1990s as minimally invasive tools enabling transdermal drug delivery, revolutionizing painless administration techniques. Advancements in nanotechnology during the early 2000s paved the way for nanorobots, which offer precise cellular-level targeting and controlled therapeutic actions. This progression from microneedles to nanorobots marks a significant evolution in biomedical engineering, enhancing drug delivery efficacy and patient outcomes.
Mechanisms of Action: How Nanorobots and Microneedles Work
Nanorobots operate by navigating through biological environments to perform targeted tasks such as drug delivery or cellular repair using sensors and actuators at the nanoscale. Microneedles penetrate the skin's outer layers to create microscopic channels that facilitate transdermal drug delivery or sampling without reaching nerve-rich deeper tissues. The precise control of nanorobot movement contrasts with the mechanical facilitation of molecule transport provided by microneedle arrays.
Precision Drug Delivery: Nanorobots vs. Microneedles
Nanorobots enable precision drug delivery by navigating at the cellular level, allowing targeted therapy with minimal side effects and enhanced efficacy. Microneedles provide a less invasive method by penetrating the skin's outer layer to deliver drugs directly into systemic circulation, though with less spatial precision compared to nanorobots. The choice between nanorobots and microneedles depends on the required targeting accuracy, drug type, and the complexity of the treatment regimen.
Biocompatibility and Safety Considerations
Nanorobots, engineered at the nanoscale, exhibit exceptional biocompatibility due to their ability to navigate cellular environments with minimal immune response, reducing cytotoxicity risks. In contrast, microneedles, typically fabricated from biocompatible polymers or metals, offer safe transdermal drug delivery but may cause localized skin irritation or infection if not properly sterilized. Safety considerations for nanorobots emphasize controlled biodegradation and precise targeting to avoid off-target effects, whereas microneedles require mechanical stability and consistent penetration depth to ensure patient safety.
Therapeutic Applications: Cancer, Diabetes, and Beyond
Nanorobots offer precise drug delivery and real-time tumor targeting in cancer therapy, improving efficacy while minimizing side effects compared to traditional microneedles. In diabetes management, microneedles enable painless insulin administration with controlled release, whereas nanorobots provide continuous glucose monitoring and responsive insulin delivery at the cellular level. Beyond cancer and diabetes, nanorobots facilitate targeted gene therapy and tissue regeneration, surpassing the capabilities of microneedles in complex therapeutic applications.
Manufacturing Challenges and Scalability
Nanorobots face significant manufacturing challenges due to their extremely small size, requiring advanced nanofabrication techniques like electron beam lithography and molecular self-assembly, which are often complex and costly. Microneedles, with their relatively larger scale, benefit from more established microfabrication methods such as photolithography and injection molding, enabling higher scalability and cost-effective mass production. The scalability of microneedle production surpasses nanorobots, making them more commercially viable for widespread clinical applications despite the precision and multifunctionality advantages nanorobots offer.
Clinical Trials and Regulatory Status
Nanorobots and microneedles represent two innovative drug delivery technologies with distinct clinical trial and regulatory landscapes. Clinical trials for nanorobots primarily focus on targeted cancer therapies, with early-phase human studies demonstrating precision drug delivery and reduced systemic toxicity, while microneedles have advanced through multiple clinical trial phases assessing vaccines, insulin delivery, and transdermal drug administration. Regulatory authorities like the FDA and EMA have granted microneedles expedited pathways based on proven safety and efficacy, whereas nanorobots remain under rigorous preclinical and investigational new drug (IND) protocols due to complex biocompatibility and manufacturing challenges.
Patient Compliance and Acceptance
Nanorobots offer precise, minimally invasive drug delivery, enhancing patient compliance through targeted therapy with reduced side effects. Microneedles provide a painless alternative to traditional injections, significantly improving patient acceptance due to their ease of use and lower discomfort. Both technologies prioritize user-friendly design, but microneedles currently show higher adoption rates in clinical settings because of established manufacturing processes and cost-effectiveness.
Future Prospects: Integrative Trends in Biomedical Devices
Nanorobots and microneedles are converging in the advancement of biomedical devices, with nanorobots offering targeted drug delivery and real-time diagnostics at the cellular level, while microneedles provide minimally invasive transdermal drug administration and vaccine delivery. The integration of nanorobotic components into microneedle arrays holds promise for enhanced precision in payload release and responsive therapeutic interventions. Emerging research focuses on hybrid systems that combine nanoscale actuation and sensing with microneedle-mediated access to improve patient outcomes and enable personalized medicine.
Targeted drug delivery
Nanorobots enable precise targeted drug delivery at the cellular level, surpassing microneedles in accuracy and controlled release within specific tissues.
Minimally invasive therapy
Nanorobots offer enhanced precision and targeted drug delivery in minimally invasive therapy compared to microneedles, which provide simpler, cost-effective skin penetration for localized treatment.
Biointerface interaction
Nanorobots exhibit superior biointerface interaction compared to microneedles due to their enhanced cellular targeting precision, dynamic responsiveness, and minimal invasiveness at the nanoscale level.
Controlled payload release
Nanorobots enable precise controlled payload release at the cellular level, surpassing microneedles in targeted drug delivery efficiency and minimizing systemic side effects.
Nanomaterial biocompatibility
Nanorobots crafted from biocompatible nanomaterials exhibit superior cellular integration and reduced immune response compared to microneedles, enhancing targeted drug delivery efficacy.
Transdermal penetration
Nanorobots achieve superior transdermal penetration compared to microneedles by precisely navigating skin layers at the cellular level, enabling targeted drug delivery with minimal invasiveness and enhanced therapeutic efficiency.
In vivo navigation
Nanorobots demonstrate superior in vivo navigation capabilities compared to microneedles due to their autonomous movement, precise targeting, and ability to traverse complex biological environments.
Microfabrication techniques
Nanorobots and microneedles are fabricated using advanced microfabrication techniques such as photolithography, laser ablation, and microelectromechanical systems (MEMS) to achieve precise structural control at micro- and nanoscale dimensions.
Smart biosensing
Nanorobots provide superior smart biosensing capabilities compared to microneedles due to their enhanced precision, real-time data processing, and targeted molecular interaction at the nanoscale level.
Precision medicine
Nanorobots enable ultra-precise drug delivery at the cellular level, surpassing microneedles in enhancing targeted precision medicine outcomes.
nanorobots vs microneedles Infographic
 njnir.com
njnir.com