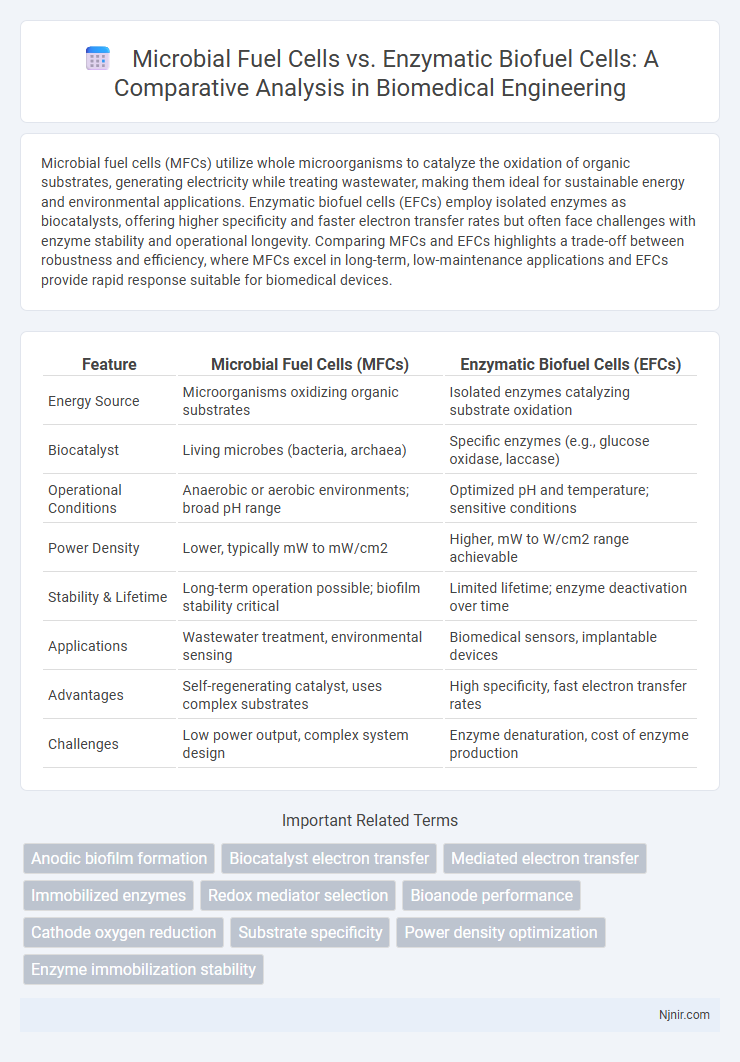
Microbial fuel cells (MFCs) utilize whole microorganisms to catalyze the oxidation of organic substrates, generating electricity while treating wastewater, making them ideal for sustainable energy and environmental applications. Enzymatic biofuel cells (EFCs) employ isolated enzymes as biocatalysts, offering higher specificity and faster electron transfer rates but often face challenges with enzyme stability and operational longevity. Comparing MFCs and EFCs highlights a trade-off between robustness and efficiency, where MFCs excel in long-term, low-maintenance applications and EFCs provide rapid response suitable for biomedical devices.
Table of Comparison
| Feature | Microbial Fuel Cells (MFCs) | Enzymatic Biofuel Cells (EFCs) |
|---|---|---|
| Energy Source | Microorganisms oxidizing organic substrates | Isolated enzymes catalyzing substrate oxidation |
| Biocatalyst | Living microbes (bacteria, archaea) | Specific enzymes (e.g., glucose oxidase, laccase) |
| Operational Conditions | Anaerobic or aerobic environments; broad pH range | Optimized pH and temperature; sensitive conditions |
| Power Density | Lower, typically mW to mW/cm2 | Higher, mW to W/cm2 range achievable |
| Stability & Lifetime | Long-term operation possible; biofilm stability critical | Limited lifetime; enzyme deactivation over time |
| Applications | Wastewater treatment, environmental sensing | Biomedical sensors, implantable devices |
| Advantages | Self-regenerating catalyst, uses complex substrates | High specificity, fast electron transfer rates |
| Challenges | Low power output, complex system design | Enzyme denaturation, cost of enzyme production |
Introduction to Biofuel Cells in Biomedical Engineering
Microbial fuel cells (MFCs) utilize electrogenic bacteria to convert organic substrates into electrical energy, whereas enzymatic biofuel cells (EFCs) employ specific enzymes as biocatalysts for electron transfer, offering higher specificity and efficiency. Both technologies serve as promising bioelectrochemical systems in biomedical engineering for powering implantable devices and biosensors by harnessing biochemical reactions under physiological conditions. Key challenges in integrating these biofuel cells into biomedical applications include biocompatibility, long-term stability, and efficient electron transfer in complex biological environments.
Fundamental Principles: Microbial vs Enzymatic Biofuel Cells
Microbial fuel cells (MFCs) generate electricity by harnessing the metabolic processes of microorganisms that oxidize organic substrates, releasing electrons transferred to an electrode via extracellular electron transfer mechanisms. Enzymatic biofuel cells (EBCs) rely on isolated enzymes as biocatalysts to oxidize specific substrates, producing electrons directly at the electrode surface with higher specificity but limited stability compared to whole-cell systems. The fundamental difference lies in MFCs utilizing intact microbial consortia for continuous electron generation from complex substrates, while EBCs depend on purified enzymes targeting specific reactions for fuel oxidation.
Electrode Materials and Biocatalysts
Microbial fuel cells (MFCs) utilize a variety of electrode materials such as carbon cloth, graphite felt, and carbon nanotubes to enhance electron transfer from living microorganisms like Geobacter sulfurreducens. Enzymatic biofuel cells (EBFCs) employ highly specific biocatalysts, namely isolated enzymes such as glucose oxidase and laccase, immobilized on electrodes made from materials like gold, graphene, or carbon nanotubes to achieve efficient catalytic oxidation and reduction reactions. The choice of electrode materials and biocatalysts directly impacts the electron transfer kinetics, stability, and overall power output of both MFCs and EBFCs.
Electron Transfer Mechanisms
Microbial fuel cells (MFCs) utilize whole microorganisms to facilitate extracellular electron transfer (EET) through direct contact or mediator molecules like phenazines and flavins, enabling electrons to flow from microbial metabolism to the anode. Enzymatic biofuel cells (EFCs) rely on isolated enzymes and employ direct electron transfer (DET) or mediated electron transfer (MET) via redox mediators such as ferrocene or quinones to shuttle electrons from enzyme active sites to the electrode. The difference in electron transfer mechanisms impacts power output and stability, with MFCs offering self-replenishing catalysts and EFCs providing higher specificity and faster electron transfer rates.
Power Output and Energy Efficiency Comparison
Microbial fuel cells (MFCs) typically generate power outputs in the range of microwatts to milliwatts per square centimeter due to the slower electron transfer rates of whole microorganisms, whereas enzymatic biofuel cells (EBFCs) can achieve higher power densities by utilizing specific enzymes that catalyze reactions more efficiently, often reaching several milliwatts per square centimeter. Energy efficiency in EBFCs is generally higher because enzymes provide direct electron transfer and minimize energy losses, while MFCs experience greater internal resistance and metabolic energy dissipation. The selectivity and catalytic specificity of enzymes in EBFCs enable more sustainable and enhanced energy conversion compared to the broader, less targeted microbial metabolism in MFCs.
Biocompatibility and Integration with Biomedical Devices
Microbial fuel cells demonstrate moderate biocompatibility due to the use of living microorganisms, which can pose challenges for seamless integration with sensitive biomedical devices. Enzymatic biofuel cells offer superior biocompatibility since isolated enzymes reduce immune responses and cellular damage, enhancing their suitability for implantable medical devices. Integration with biomedical devices favors enzymatic biofuel cells because their smaller size, stability, and specificity enable efficient power generation in physiological conditions without compromising device functionality.
Longevity and Operational Stability
Microbial fuel cells (MFCs) generally exhibit greater longevity and operational stability due to the natural proliferation and self-regeneration of microorganisms, enabling sustained electron generation over extended periods. In contrast, enzymatic biofuel cells typically face limited lifespans as enzyme activity diminishes rapidly because of denaturation and environmental sensitivity, restricting long-term functionality. Advances in immobilization techniques and electrode materials aim to improve enzymatic biofuel cells' stability but still lag behind the durable biofilm formation seen in microbial fuel cells.
Challenges in In Vivo Applications
Microbial fuel cells (MFCs) face challenges in in vivo applications due to biocompatibility issues, maintaining microbial viability, and efficient electron transfer within complex biological environments. Enzymatic biofuel cells (EBCs) struggle with enzyme stability, limited operational lifespan, and maintaining catalytic activity under physiological conditions. Both systems require advancements in material engineering and immobilization techniques to overcome biofouling and immune response hurdles.
Recent Advances and Innovations
Recent advances in microbial fuel cells (MFCs) include enhanced electrode materials like graphene-based composites that boost electron transfer efficiency and microbial activity, significantly increasing power density. Innovations in enzymatic biofuel cells (EBFCs) focus on immobilizing multi-enzyme cascades on nanostructured electrodes, improving catalytic stability and substrate specificity under physiological conditions. Both technologies benefit from integrating bio-inspired nanomaterials and advanced bioelectrochemical engineering to optimize energy conversion and scalability for practical applications.
Future Perspectives in Biomedical Applications
Microbial fuel cells (MFCs) offer sustainable energy generation through the catalytic activity of microorganisms, presenting promising future perspectives in implantable biomedical devices due to their ability to generate power from bodily fluids. Enzymatic biofuel cells (EBCs) utilize highly specific enzymes for efficient electron transfer, enabling miniaturized power sources with enhanced biocompatibility and targeted applications in biosensors and drug delivery systems. Advancements in electrode materials, enzyme engineering, and microbial consortia optimization are expected to drive the integration of both MFCs and EBCs in next-generation biomedical implants with improved longevity and performance.
Anodic biofilm formation
Anodic biofilm formation in microbial fuel cells involves complex microbial consortia that enhance electron transfer, whereas enzymatic biofuel cells rely on isolated enzymes immobilized on the anode, resulting in less robust biofilm development and electron transfer kinetics.
Biocatalyst electron transfer
Microbial fuel cells utilize whole microorganisms for indirect electron transfer through conductive pili or mediators, whereas enzymatic biofuel cells rely on isolated enzymes enabling direct electron transfer at the electrode interface for enhanced specificity and efficiency.
Mediated electron transfer
Microbial fuel cells utilize mediators such as redox-active compounds for electron transfer between microbial enzymes and electrodes, while enzymatic biofuel cells rely on mediator molecules to facilitate electron transfer directly from isolated enzymes to electrodes, enhancing efficiency and specificity.
Immobilized enzymes
Immobilized enzymes in enzymatic biofuel cells enhance electron transfer efficiency and operational stability compared to microbial fuel cells, which rely on whole-cell metabolism.
Redox mediator selection
Redox mediator selection in microbial fuel cells prioritizes biocompatibility and electron transfer efficiency within complex microbial environments, whereas enzymatic biofuel cells require mediators with high specificity and stability to optimize direct electron transfer from isolated enzymes.
Bioanode performance
Microbial fuel cells demonstrate enhanced bioanode performance through diverse microbial community activity enabling higher current densities, while enzymatic biofuel cells offer specificity and faster electron transfer rates but often suffer from enzyme instability and limited operational lifespan.
Cathode oxygen reduction
Microbial fuel cells typically utilize microbial catalysts for cathode oxygen reduction with moderate efficiency, whereas enzymatic biofuel cells employ specific enzymes, such as laccase or bilirubin oxidase, enabling higher catalytic activity and lower overpotential for oxygen reduction at the cathode.
Substrate specificity
Microbial fuel cells utilize a broad range of substrates through diverse microbial metabolism, while enzymatic biofuel cells exhibit high substrate specificity due to selective enzyme activity.
Power density optimization
Microbial fuel cells achieve power density optimization through microbial consortium engineering and electrode surface modification, while enzymatic biofuel cells enhance power density via enzyme immobilization techniques and mediator optimization.
Enzyme immobilization stability
Enzymatic biofuel cells demonstrate enhanced enzyme immobilization stability through advanced matrix materials and cross-linking techniques, surpassing microbial fuel cells in maintaining catalytic activity over extended periods.
Microbial fuel cells vs Enzymatic biofuel cells Infographic
 njnir.com
njnir.com