Exosome delivery offers superior biocompatibility and targeted cellular communication compared to synthetic nanoparticles, reducing immune clearance and enhancing therapeutic efficacy. Their innate ability to cross biological barriers facilitates efficient drug delivery to hard-to-reach tissues. Unlike nanoparticles, exosomes naturally carry complex biomolecules, promoting more precise and controlled release mechanisms in biomedical applications.
Table of Comparison
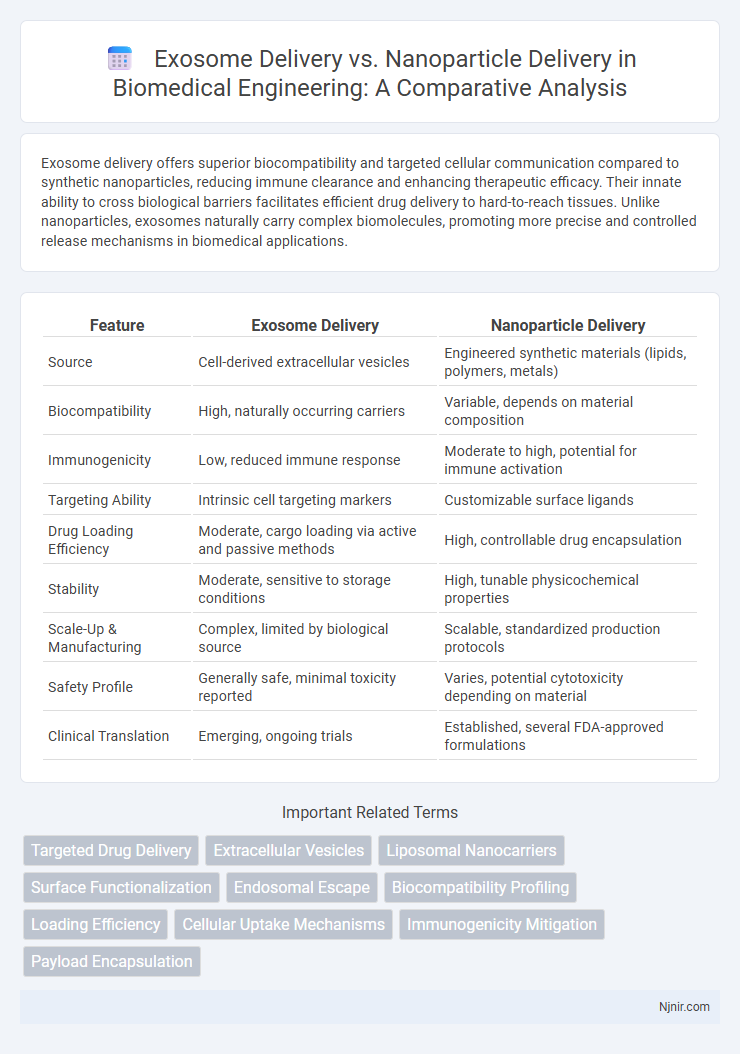
| Feature | Exosome Delivery | Nanoparticle Delivery |
|---|---|---|
| Source | Cell-derived extracellular vesicles | Engineered synthetic materials (lipids, polymers, metals) |
| Biocompatibility | High, naturally occurring carriers | Variable, depends on material composition |
| Immunogenicity | Low, reduced immune response | Moderate to high, potential for immune activation |
| Targeting Ability | Intrinsic cell targeting markers | Customizable surface ligands |
| Drug Loading Efficiency | Moderate, cargo loading via active and passive methods | High, controllable drug encapsulation |
| Stability | Moderate, sensitive to storage conditions | High, tunable physicochemical properties |
| Scale-Up & Manufacturing | Complex, limited by biological source | Scalable, standardized production protocols |
| Safety Profile | Generally safe, minimal toxicity reported | Varies, potential cytotoxicity depending on material |
| Clinical Translation | Emerging, ongoing trials | Established, several FDA-approved formulations |
Introduction to Exosome and Nanoparticle Delivery
Exosome delivery utilizes naturally derived extracellular vesicles that facilitate targeted cellular communication and cargo transport, enhancing biocompatibility and reducing immune clearance. Nanoparticle delivery systems are engineered synthetic materials designed to optimize drug stability, controlled release, and targeted delivery but may face challenges related to toxicity and clearance. Both platforms play critical roles in advancing precision medicine, with exosomes offering inherent biological advantages and nanoparticles enabling customizable therapeutic payloads.
Mechanisms of Exosome-Mediated Delivery
Exosome-mediated delivery utilizes cell-derived vesicles that naturally transport biomolecules through membrane fusion and endocytosis pathways, ensuring efficient payload transfer to target cells. Unlike synthetic nanoparticles, exosomes possess inherent surface proteins that facilitate specific cellular recognition and uptake, enhancing delivery precision and biocompatibility. Their ability to evade immune detection and cross biological barriers contributes to superior therapeutic efficacy in drug and gene delivery applications.
Nanoparticle Delivery: Principles and Methods
Nanoparticle delivery utilizes engineered particles typically ranging from 1 to 100 nanometers to transport therapeutic agents directly to target cells, enhancing drug bioavailability and reducing systemic toxicity. Common methods include liposomes, polymeric nanoparticles, and inorganic nanoparticles, each offering unique properties such as controlled release, surface modification for targeting, and biocompatibility. Key principles involve optimizing particle size, surface charge, and ligand attachment to ensure efficient cellular uptake and precise drug delivery in treatments like cancer therapy and gene editing.
Comparative Efficiency in Drug Encapsulation
Exosome delivery systems exhibit superior drug encapsulation efficiency compared to conventional nanoparticles due to their natural lipid bilayer structure and inherent biocompatibility, which facilitate higher payload stability and targeted release. Nanoparticles often face challenges such as limited loading capacity and premature drug leakage, reducing therapeutic efficacy. Studies demonstrate encapsulation efficiencies for exosomes ranging from 60% to 85%, surpassing many synthetic nanoparticles that typically achieve 40% to 70%, highlighting exosomes as a more efficient carrier for drug delivery applications.
Targeting Capabilities: Exosomes vs Nanoparticles
Exosome delivery systems exhibit superior targeting capabilities compared to traditional nanoparticles due to their inherent surface proteins and lipid composition that facilitate natural cell recognition and uptake. Unlike synthetic nanoparticles, exosomes can traverse biological barriers effectively and deliver cargo with high precision to specific cells or tissues, reducing off-target effects. The biomimetic nature of exosomes enhances biodistribution and cellular communication, making them a promising vehicle for targeted drug delivery in therapeutic applications.
Biocompatibility and Immune Response
Exosome delivery systems exhibit superior biocompatibility due to their natural origin and ability to evade immune recognition, minimizing adverse immune responses compared to synthetic nanoparticles. Nanoparticles often trigger immune activation and inflammatory reactions because of their foreign surface properties and potential cytotoxicity. The inherent lipid bilayer and surface proteins of exosomes facilitate targeted delivery with reduced immunogenicity, enhancing therapeutic efficacy in clinical applications.
Scalability and Manufacturing Challenges
Exosome delivery faces significant scalability and manufacturing challenges due to the complexity of isolating and purifying extracellular vesicles at large volumes with consistent quality. Nanoparticle delivery systems benefit from more established, scalable chemical synthesis methods allowing for controlled production and easier quality control. However, ensuring biocompatibility and reproducibility remains a hurdle for both platforms in clinical-grade manufacturing.
Therapeutic Applications in Biomedical Engineering
Exosome delivery systems offer enhanced biocompatibility and targeted drug transport compared to synthetic nanoparticles, leveraging their natural origin and ability to cross biological barriers for improved therapeutic efficacy. Nanoparticle delivery enables precise control over size, surface modification, and drug release kinetics, facilitating customized treatments for cancer, gene therapy, and regenerative medicine. Both platforms show promise in biomedical engineering by enabling site-specific delivery, reducing systemic toxicity, and enhancing the stability of therapeutic molecules.
Clinical Translation: Current Progress and Barriers
Exosome delivery systems demonstrate enhanced biocompatibility and targeted delivery in clinical translation, with multiple ongoing trials highlighting their potential in precision medicine. Nanoparticle delivery platforms, while advancing through scalable manufacturing and regulatory approvals, still face challenges relating to immunogenicity and off-target effects. Both modalities require optimized formulation strategies and rigorous safety evaluations to overcome barriers for widespread clinical adoption in drug delivery and therapeutic applications.
Future Perspectives in Delivery System Development
Exosome delivery systems offer superior biocompatibility and intrinsic targeting capabilities compared to traditional nanoparticle delivery, which often faces challenges in immune clearance and limited cellular uptake. Future perspectives emphasize engineering exosomes for enhanced cargo loading efficiency and surface modification to achieve precise tissue targeting and controlled release. Integration of synthetic biology and advanced nanotechnology aims to create hybrid delivery platforms combining the stability of nanoparticles with the biological functionality of exosomes for next-generation therapeutics.
Targeted Drug Delivery
Exosome delivery offers superior targeted drug delivery compared to nanoparticles due to its natural biocompatibility, cell-specific targeting capabilities, and ability to evade immune clearance.
Extracellular Vesicles
Extracellular vesicles, specifically exosomes, offer targeted delivery with enhanced biocompatibility and reduced immunogenicity compared to synthetic nanoparticles in drug and gene therapy applications.
Liposomal Nanocarriers
Liposomal nanocarriers offer enhanced biocompatibility and targeted drug delivery compared to exosome delivery by providing controlled release and improved stability for therapeutic agents.
Surface Functionalization
Exosome delivery systems exhibit superior surface functionalization capabilities compared to nanoparticle delivery, enabling enhanced biocompatibility, targeted cellular uptake, and reduced immunogenicity for precise therapeutic applications.
Endosomal Escape
Exosome delivery demonstrates superior endosomal escape efficiency compared to nanoparticle delivery, enhancing the intracellular release and bioavailability of therapeutic agents.
Biocompatibility Profiling
Exosome delivery systems demonstrate superior biocompatibility profiling compared to synthetic nanoparticles due to their natural origin, reduced immunogenicity, and enhanced cellular uptake.
Loading Efficiency
Exosome delivery demonstrates higher loading efficiency compared to nanoparticle delivery due to its natural membrane fusion capabilities and selective cargo packaging mechanisms.
Cellular Uptake Mechanisms
Exosome delivery exploits natural membrane fusion and receptor-mediated endocytosis for efficient cellular uptake, whereas nanoparticle delivery primarily relies on endocytic pathways such as clathrin-mediated and caveolae-mediated endocytosis with variable efficiency.
Immunogenicity Mitigation
Exosome delivery exhibits lower immunogenicity than nanoparticle delivery due to its natural origin and intrinsic biocompatibility, enhancing immune system evasion and reducing inflammatory responses.
Payload Encapsulation
Exosome delivery demonstrates superior payload encapsulation efficiency and biocompatibility compared to synthetic nanoparticles, enabling enhanced targeted therapeutic cargo protection and controlled release.
exosome delivery vs nanoparticle delivery Infographic
 njnir.com
njnir.com