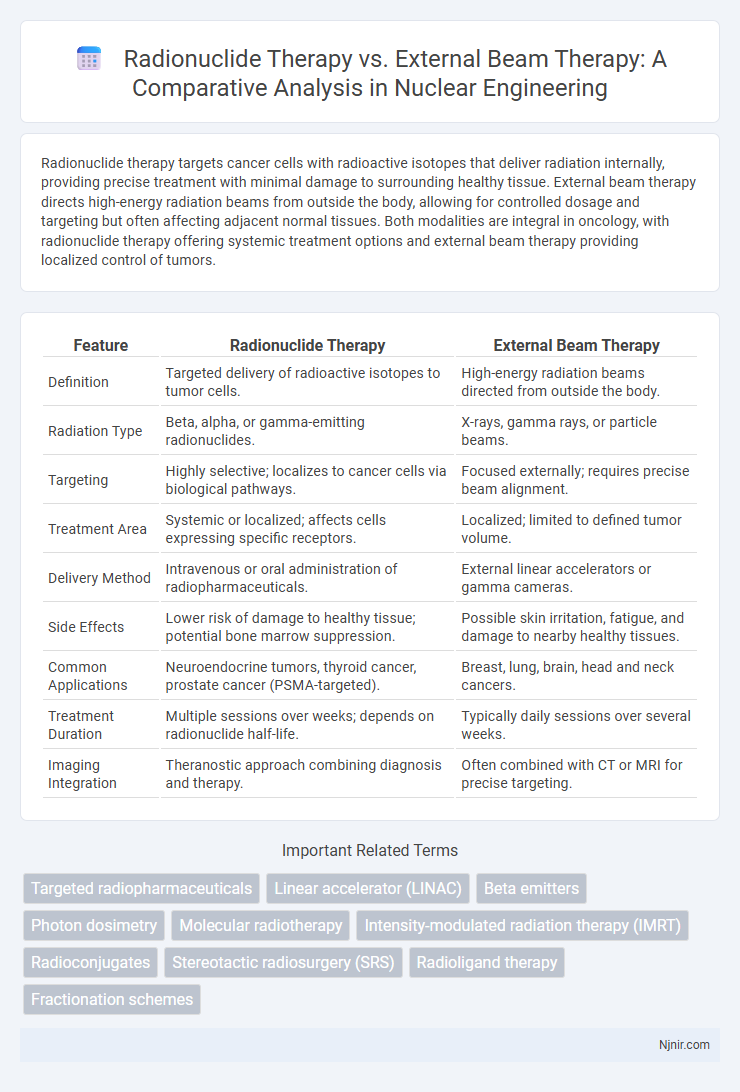
Radionuclide therapy targets cancer cells with radioactive isotopes that deliver radiation internally, providing precise treatment with minimal damage to surrounding healthy tissue. External beam therapy directs high-energy radiation beams from outside the body, allowing for controlled dosage and targeting but often affecting adjacent normal tissues. Both modalities are integral in oncology, with radionuclide therapy offering systemic treatment options and external beam therapy providing localized control of tumors.
Table of Comparison
| Feature | Radionuclide Therapy | External Beam Therapy |
|---|---|---|
| Definition | Targeted delivery of radioactive isotopes to tumor cells. | High-energy radiation beams directed from outside the body. |
| Radiation Type | Beta, alpha, or gamma-emitting radionuclides. | X-rays, gamma rays, or particle beams. |
| Targeting | Highly selective; localizes to cancer cells via biological pathways. | Focused externally; requires precise beam alignment. |
| Treatment Area | Systemic or localized; affects cells expressing specific receptors. | Localized; limited to defined tumor volume. |
| Delivery Method | Intravenous or oral administration of radiopharmaceuticals. | External linear accelerators or gamma cameras. |
| Side Effects | Lower risk of damage to healthy tissue; potential bone marrow suppression. | Possible skin irritation, fatigue, and damage to nearby healthy tissues. |
| Common Applications | Neuroendocrine tumors, thyroid cancer, prostate cancer (PSMA-targeted). | Breast, lung, brain, head and neck cancers. |
| Treatment Duration | Multiple sessions over weeks; depends on radionuclide half-life. | Typically daily sessions over several weeks. |
| Imaging Integration | Theranostic approach combining diagnosis and therapy. | Often combined with CT or MRI for precise targeting. |
Introduction to Radionuclide and External Beam Therapy
Radionuclide therapy involves administering radioactive isotopes directly to target cancer cells, delivering localized radiation from within the body, enhancing precision in destroying malignant tissues. External beam therapy uses high-energy X-rays or particle beams directed from outside the body to irradiate tumors, allowing for controlled dose distribution and sparing surrounding healthy tissues. Both therapies play crucial roles in oncologic treatment, with radionuclide therapy offering systemic targeting and external beam therapy providing versatile, adjustable radiation delivery.
Mechanisms of Action: Radionuclide vs External Beam Therapy
Radionuclide therapy delivers targeted radiation by using radioactive isotopes that selectively bind to cancer cells, emitting ionizing radiation internally to damage tumor DNA. External beam therapy employs high-energy X-rays or particles directed from outside the body, focusing radiation on tumor sites to induce DNA breaks in cancer cells. The main distinction lies in radionuclide therapy's systemic or localized internal radiation versus external beam therapy's precise external irradiation, influencing treatment specificity and tissue exposure.
Types of Radionuclides and Isotopes Used
Radionuclide therapy employs specific radioactive isotopes such as Iodine-131 for thyroid cancer and Lutetium-177 for neuroendocrine tumors, targeting cancer cells through systemic administration. External beam therapy relies on high-energy X-rays or proton beams directed precisely at tumors, without introducing radioactive materials into the body. The choice of radionuclides like Yttrium-90 and Radium-223 allows for selective internal radiation, contrasting with the external targeting mechanism of photons and charged particles in external beam radiation.
Treatment Planning and Dosimetry Comparison
Radionuclide therapy utilizes radiopharmaceuticals that deliver targeted radiation doses at the cellular level, requiring individualized treatment planning based on patient-specific biodistribution and pharmacokinetics, often assessed via SPECT or PET imaging. In contrast, external beam therapy relies on precise imaging modalities like CT or MRI to map tumor volumes for spatially optimized dose distributions, employing advanced algorithms to minimize damage to surrounding healthy tissues. Dosimetry in radionuclide therapy involves calculating absorbed doses considering biological clearance and organ-specific uptake, whereas external beam dosimetry depends on physical beam parameters and patient anatomy for accurate dose delivery.
Clinical Indications and Applications
Radionuclide therapy is primarily indicated for the treatment of metastatic or inoperable cancers such as thyroid carcinoma, neuroendocrine tumors, and metastatic prostate cancer through targeted delivery of radioactive isotopes like I-131, Lu-177, or Ra-223. External beam therapy is widely applied in localized tumors including head and neck cancers, breast cancer, and lung cancer, utilizing high-energy X-rays or proton beams for precise tumor targeting while sparing adjacent normal tissues. Both modalities have distinct clinical applications with radionuclide therapy offering systemic treatment benefits and external beam therapy providing localized control with well-established protocols for dosage and fractionation.
Efficacy in Targeted Tumor Control
Radionuclide therapy delivers targeted radiation at a cellular level by using radiolabeled molecules that selectively bind to tumor cells, resulting in highly effective tumor control with minimal damage to surrounding healthy tissue. External beam therapy provides focused high-energy beams that effectively reduce tumor size but may affect adjacent normal tissue due to less specificity. Clinical studies demonstrate that radionuclide therapy offers improved efficacy in treating metastatic and difficult-to-reach tumors compared to conventional external beam radiation.
Side Effects and Toxicity Profiles
Radionuclide therapy typically causes targeted toxicity, resulting in fewer systemic side effects, with common issues including mild hematologic toxicity and transient fatigue. External beam therapy often leads to localized side effects such as skin irritation, mucositis, and organ-specific damage depending on the treatment site, occasionally causing more pronounced acute and chronic toxicities. Both modalities require careful dose management to minimize toxicity while maximizing therapeutic efficacy.
Patient Selection Criteria
Radionuclide therapy is typically selected for patients with metastatic or multifocal disease due to its systemic delivery and ability to target specific molecular markers, whereas external beam therapy (EBT) is preferred for localized tumors given its precise targeting and dose control. Patient factors such as tumor type, stage, and expression of target receptors significantly influence eligibility for radionuclide therapy, while EBT suitability depends on tumor size, location, and surrounding normal tissue tolerance. Comprehensive evaluation including imaging, molecular profiling, and organ function tests guides the optimal choice between radionuclide therapy and external beam therapy.
Recent Advances in Radiotherapy Technologies
Recent advances in radiotherapy technologies have enhanced the precision and efficacy of both radionuclide therapy and external beam therapy. Radionuclide therapy leverages targeted delivery of radioactive isotopes like Lutetium-177 and Yttrium-90, optimizing tumor-specific radiotoxicity while sparing healthy tissue. External beam therapy innovations such as intensity-modulated radiation therapy (IMRT) and proton beam therapy enable superior dose conformity and reduced collateral damage, improving outcomes in complex tumor sites.
Future Perspectives and Research Directions
Radionuclide therapy offers targeted treatment by delivering radioactive isotopes directly to cancer cells, which is being enhanced through advances in molecular imaging and novel radiopharmaceuticals for personalized oncology. Research is focusing on optimizing isotope selection, dosimetry accuracy, and combining radionuclide therapy with immunotherapy or gene therapy to improve efficacy and reduce toxicity. Emerging technologies such as alpha-emitters and nanocarriers are driving the future direction towards more precise and effective cancer treatment modalities compared to traditional external beam therapy.
Targeted radiopharmaceuticals
Targeted radiopharmaceuticals in radionuclide therapy deliver selective radiation to cancer cells, enhancing precision and minimizing damage compared to the broader exposure of external beam therapy.
Linear accelerator (LINAC)
Linear accelerator (LINAC) external beam therapy delivers high-energy X-rays precisely to tumors, offering targeted treatment compared to radionuclide therapy, which uses radioactive substances to irradiate cancer cells internally.
Beta emitters
Radionuclide therapy using beta emitters delivers targeted radiation to cancer cells with minimal damage to surrounding tissues, while external beam therapy relies on external radiation sources that can affect a broader area but offer precise dosage control.
Photon dosimetry
Photon dosimetry in radionuclide therapy precisely targets intracellular radioactive sources, offering superior dose conformity and reduced exposure to surrounding tissues compared to external beam therapy.
Molecular radiotherapy
Molecular radiotherapy delivers targeted radionuclide therapy by administering radiopharmaceuticals that selectively accumulate in cancer cells, offering precise treatment compared to the broader tissue exposure of external beam radiotherapy.
Intensity-modulated radiation therapy (IMRT)
Intensity-modulated radiation therapy (IMRT) offers precise external beam radiation that minimizes damage to surrounding tissues compared to radionuclide therapy by modulating beam intensity for targeted tumor treatment.
Radioconjugates
Radioconjugates in radionuclide therapy deliver targeted radiation to tumor cells with minimal damage to surrounding tissue, offering a more precise alternative to the broader, less selective radiation exposure of external beam therapy.
Stereotactic radiosurgery (SRS)
Stereotactic radiosurgery (SRS) delivers high-dose, precisely targeted external beam radiation for localized tumors, contrasting with radionuclide therapy's systemic approach using radioactive isotopes for dispersed cancer cells.
Radioligand therapy
Radioligand therapy, a targeted form of radionuclide therapy, delivers radioactive isotopes directly to cancer cells, offering higher precision and reduced damage to surrounding tissues compared to conventional external beam radiation therapy.
Fractionation schemes
Radionuclide therapy delivers targeted radiation internally without fractionation, while external beam therapy employs precise fractionation schemes, typically administering daily doses over several weeks to maximize tumor control and minimize normal tissue damage.
radionuclide therapy vs external beam therapy Infographic
 njnir.com
njnir.com