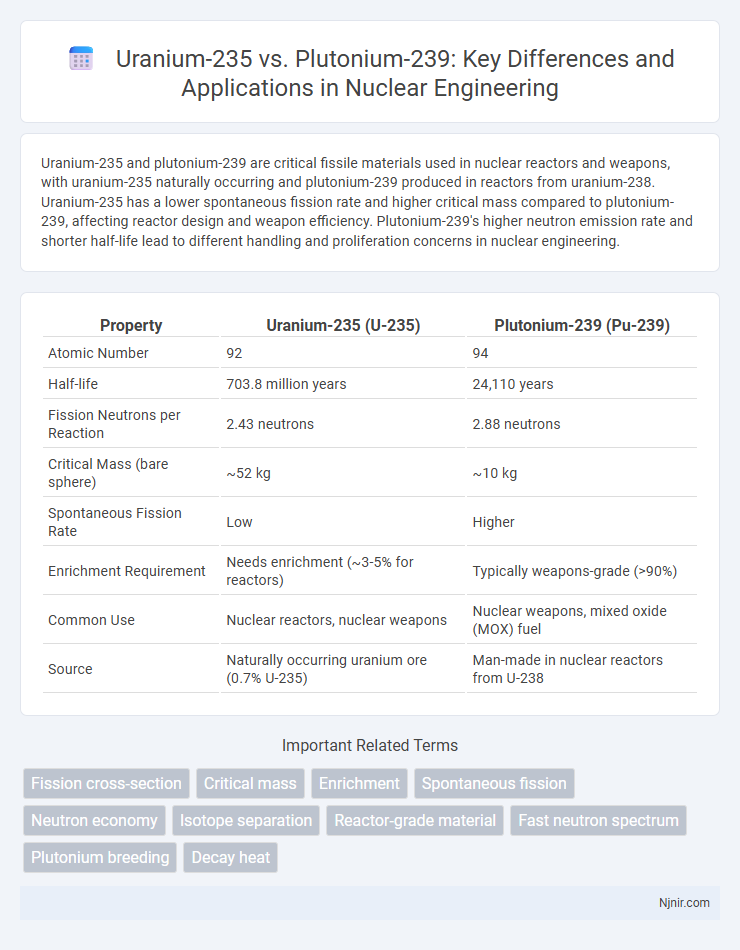
Uranium-235 and plutonium-239 are critical fissile materials used in nuclear reactors and weapons, with uranium-235 naturally occurring and plutonium-239 produced in reactors from uranium-238. Uranium-235 has a lower spontaneous fission rate and higher critical mass compared to plutonium-239, affecting reactor design and weapon efficiency. Plutonium-239's higher neutron emission rate and shorter half-life lead to different handling and proliferation concerns in nuclear engineering.
Table of Comparison
| Property | Uranium-235 (U-235) | Plutonium-239 (Pu-239) |
|---|---|---|
| Atomic Number | 92 | 94 |
| Half-life | 703.8 million years | 24,110 years |
| Fission Neutrons per Reaction | 2.43 neutrons | 2.88 neutrons |
| Critical Mass (bare sphere) | ~52 kg | ~10 kg |
| Spontaneous Fission Rate | Low | Higher |
| Enrichment Requirement | Needs enrichment (~3-5% for reactors) | Typically weapons-grade (>90%) |
| Common Use | Nuclear reactors, nuclear weapons | Nuclear weapons, mixed oxide (MOX) fuel |
| Source | Naturally occurring uranium ore (0.7% U-235) | Man-made in nuclear reactors from U-238 |
Overview of Uranium-235 and Plutonium-239
Uranium-235 is a naturally occurring fissile isotope used primarily in nuclear reactors and atomic bombs due to its ability to sustain a chain reaction. Plutonium-239, produced artificially in nuclear reactors from Uranium-238, exhibits a higher neutron yield and greater suitability for compact nuclear weapons. Both isotopes play critical roles in nuclear energy and weaponry, distinguished by their origin, isotopic properties, and nuclear fission characteristics.
Physical and Chemical Properties Comparison
Uranium-235 has a density of 19.1 g/cm3 and a melting point of 1,132degC, while plutonium-239 is denser at 19.8 g/cm3 with a melting point around 640degC. Chemically, uranium-235 tends to form stable oxidation states +4 and +6, whereas plutonium-239 exhibits multiple oxidation states ranging from +3 to +7, showing greater chemical versatility. Both isotopes are highly reactive with halogens and acids but plutonium's complex redox behavior influences its handling and storage requirements.
Natural Occurrence and Production Methods
Uranium-235 naturally occurs in uranium ore at approximately 0.7% abundance and is primarily extracted through mining and uranium enrichment processes. Plutonium-239 does not occur naturally in significant amounts and is produced artificially in nuclear reactors by irradiating uranium-238 with neutrons. Both isotopes are critical for nuclear fuel and weapons, with uranium-235 being mined directly and plutonium-239 generated through neutron capture and subsequent beta decay.
Fission Characteristics and Reactor Suitability
Uranium-235 exhibits a higher neutron capture cross-section for thermal neutrons, making it highly efficient for fission in light-water reactors. Plutonium-239, with its greater fissile potential and faster neutron spectrum adaptability, is preferred in fast breeder reactors for efficient fuel breeding and sustainability. Both isotopes differ in their delayed neutron fractions, with U-235 providing more favorable kinetics for reactor control and safety.
Enrichment and Fuel Fabrication Techniques
Uranium-235 enrichment involves processes like gas centrifugation and gaseous diffusion to increase the isotope concentration from 0.7% to 3-5% for reactor fuel, while plutonium-239 is typically separated from spent nuclear fuel through chemical reprocessing techniques such as PUREX. Fuel fabrication for uranium-235 requires conversion into uranium dioxide (UO2) pellets, which are then assembled into fuel rods, whereas plutonium-239 is formed into mixed oxide (MOX) fuel pellets by blending with depleted uranium dioxide, offering an alternative recycling pathway. The distinct isotopic compositions and chemical behaviors dictate specialized enrichment and fabrication methods tailored to each fissile material's nuclear and physical properties.
Weapons-Grade Considerations and Proliferation Risks
Uranium-235 and plutonium-239 are critical isotopes in nuclear weapons development due to their fissile properties, with uranium-235 typically enriched above 90% for weapons-grade material, while weapons-grade plutonium contains over 93% plutonium-239. Plutonium-239's higher spontaneous neutron emission and shorter half-life of 24,100 years complicate detection but increase proliferation risks compared to uranium-235, which has a half-life of 703.8 million years. The distinct production pathways--uranium enrichment versus plutonium reactor irradiation--pose unique challenges for monitoring and controlling illicit nuclear weapons development.
Radioactive Decay and Waste Management
Uranium-235 undergoes alpha decay with a half-life of about 703.8 million years, producing thorium-231 and releasing relatively lower levels of long-lived radioactive waste compared to plutonium-239. Plutonium-239 decays primarily through alpha emission with a half-life of 24,100 years, resulting in more radiotoxic isotopes that complicate waste management due to its higher neutron emission and alpha activity. Effective waste management strategies for plutonium-239 require enhanced containment and shielding measures to mitigate its long-term radiological hazards and potential for proliferation.
Applications in Civil Nuclear Energy
Uranium-235 is the primary fuel used in most commercial nuclear reactors due to its ability to sustain a controlled fission chain reaction with thermal neutrons, making it ideal for electricity generation in light water reactors. Plutonium-239, produced through neutron capture in uranium-238, serves as both a valuable fuel in mixed oxide (MOX) fuel and a key component in fast breeder reactors, which enhance fuel efficiency by generating more fissile material. The utilization of plutonium-239 helps extend nuclear fuel resources and supports the recycling of spent nuclear fuel in advanced reactor systems.
Safety, Handling, and Regulatory Challenges
Uranium-235 requires stringent criticality safety protocols due to its significant neutron emission but is comparatively more stable chemically than plutonium-239, which poses higher radiotoxicity and contamination risks during handling. Plutonium-239's alpha decay and pyrophoric nature necessitate specialized containment measures and rigorous decontamination procedures to prevent inhalation hazards and environmental release. Regulatory frameworks impose tougher security and transportation controls on plutonium-239 because of its greater proliferation potential and long-term environmental persistence.
Future Prospects and Technological Innovations
Uranium-235 and Plutonium-239 play pivotal roles in the future of nuclear energy, with advancements in fast breeder reactors enhancing the efficient breeding of Plutonium-239 from Uranium-238, thereby extending fuel sustainability. Innovations in small modular reactors (SMRs) are designed to optimize the use of uranium-235, offering improved safety features and lower proliferation risks. Emerging technologies like advanced reprocessing techniques aim to recycle Plutonium-239, minimizing nuclear waste and bolstering the economic viability of thorium and uranium fuel cycles.
Fission cross-section
Uranium-235 has a thermal neutron fission cross-section of about 585 barns, whereas Plutonium-239 exhibits a higher thermal fission cross-section near 750 barns, making Pu-239 more efficient for sustaining nuclear chain reactions.
Critical mass
Uranium-235 has a critical mass of approximately 52 kilograms, while plutonium-239 possesses a significantly lower critical mass of about 10 kilograms, making plutonium-239 more efficient for nuclear chain reactions.
Enrichment
Uranium-235 typically requires enrichment from 0.7% to 3-5% for reactor fuel, whereas plutonium-239 is produced via neutron capture in reactors without traditional enrichment processes.
Spontaneous fission
Uranium-235 exhibits a significantly lower spontaneous fission rate compared to plutonium-239, making plutonium-239 more prone to neutron emission without external neutron initiation.
Neutron economy
Uranium-235 has a superior neutron economy compared to plutonium-239 due to its higher average number of neutrons released per fission and lower neutron capture cross-section, resulting in more efficient sustained nuclear chain reactions.
Isotope separation
Uranium-235 requires complex gaseous diffusion or centrifuge isotope separation techniques due to its low natural abundance, whereas plutonium-239 is typically produced in reactors and separated chemically rather than isotopically.
Reactor-grade material
Reactor-grade uranium-235 typically contains 3-5% U-235 isotopes for efficient energy production, whereas reactor-grade plutonium-239 is characterized by a mix of isotopes with less than 80% Pu-239, affecting its suitability for nuclear fuel.
Fast neutron spectrum
Uranium-235 and plutonium-239 both sustain fission in a fast neutron spectrum, but plutonium-239 exhibits a higher fission cross-section and greater neutron yield, making it more efficient for fast reactor applications.
Plutonium breeding
Plutonium-239 is bred from Uranium-238 through neutron capture in breeder reactors, enabling efficient nuclear fuel recycling and extending fuel supply compared to using Uranium-235 alone.
Decay heat
Uranium-235 produces lower decay heat compared to plutonium-239, making plutonium-239 a more challenging isotope for post-reactor cooling and waste management.
uranium-235 vs plutonium-239 Infographic
 njnir.com
njnir.com