Supercritical fluids exhibit unique properties that combine gas-like diffusivity and liquid-like density, enhancing mass transfer and reaction rates in chemical engineering processes. Subcritical fluids, operating below the critical temperature and pressure, maintain separate liquid and gas phases, often resulting in slower kinetics and limited solubility for certain compounds. Supercritical fluids are preferred in applications such as extraction, chromatography, and green solvent processes due to their tunable solvating power and reduced environmental impact.
Table of Comparison
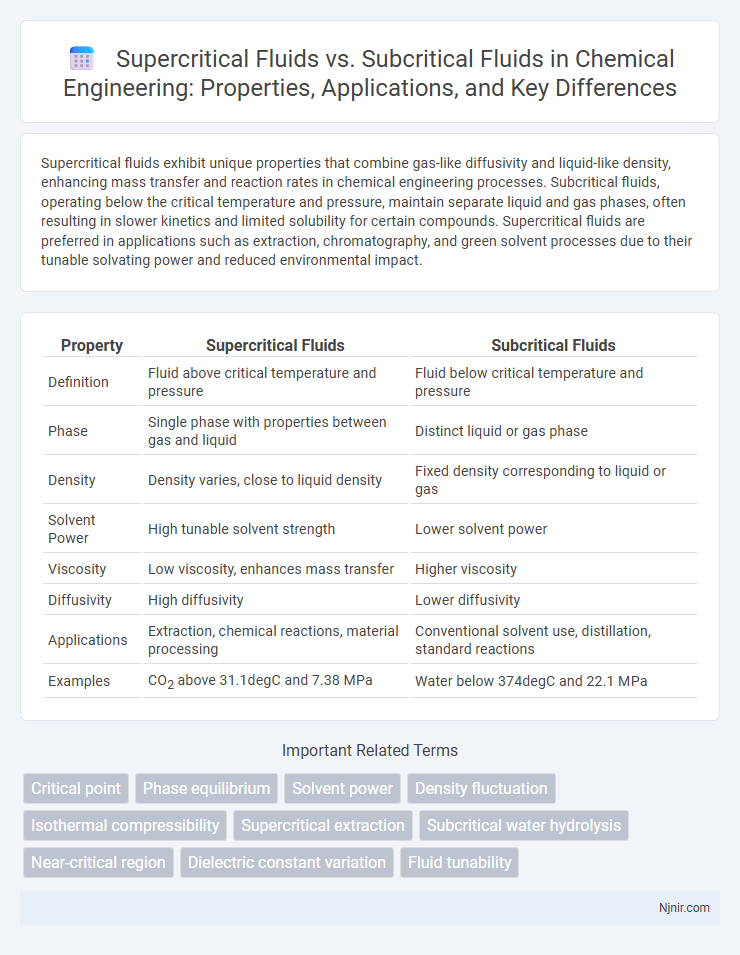
| Property | Supercritical Fluids | Subcritical Fluids |
|---|---|---|
| Definition | Fluid above critical temperature and pressure | Fluid below critical temperature and pressure |
| Phase | Single phase with properties between gas and liquid | Distinct liquid or gas phase |
| Density | Density varies, close to liquid density | Fixed density corresponding to liquid or gas |
| Solvent Power | High tunable solvent strength | Lower solvent power |
| Viscosity | Low viscosity, enhances mass transfer | Higher viscosity |
| Diffusivity | High diffusivity | Lower diffusivity |
| Applications | Extraction, chemical reactions, material processing | Conventional solvent use, distillation, standard reactions |
| Examples | CO2 above 31.1degC and 7.38 MPa | Water below 374degC and 22.1 MPa |
Introduction to Supercritical and Subcritical Fluids
Supercritical fluids exist above their critical temperature and pressure, exhibiting unique properties that combine liquid-like density with gas-like diffusion rates, making them highly effective for extraction and reaction processes. Subcritical fluids remain below the critical point, maintaining distinct liquid or gas phases with more limited solubility and diffusivity compared to supercritical states but offering controlled solvent properties for specific applications. The critical point parameters, such as carbon dioxide's critical temperature of 31.1degC and pressure of 73.8 bar, serve as benchmarks for distinguishing supercritical from subcritical phases in industrial and laboratory settings.
Fundamental Properties and Definitions
Supercritical fluids exist above their critical temperature and pressure, exhibiting unique properties that combine liquid-like density with gas-like diffusivity, enabling enhanced solvation and mass transfer capabilities. Subcritical fluids operate below the critical point, maintaining distinct liquid or gas phases with lower diffusivity and solvation power compared to supercritical fluids. The critical point marks the boundary where fluid density, viscosity, and surface tension converge to allow supercritical behavior, essential for applications in extraction, chromatography, and material processing.
Thermodynamic Behavior: Pressure and Temperature Effects
Supercritical fluids exist above their critical temperature and pressure, exhibiting unique properties such as enhanced diffusivity and solvating power due to the lack of distinct liquid and gas phases. In contrast, subcritical fluids maintain separate liquid and vapor phases, with thermodynamic behavior highly sensitive to temperature and pressure variations within phase boundaries. The transition from subcritical to supercritical conditions results in drastic changes in density and compressibility, enabling superior mass transfer and extraction efficiency in processes like supercritical fluid extraction (SFE).
Solvent Power and Solubility Differences
Supercritical fluids exhibit superior solvent power compared to subcritical fluids due to their unique density and diffusivity properties, allowing enhanced solubility of a wide range of solutes. The tunable density of supercritical fluids, often above the critical temperature and pressure, enables selective solvation, whereas subcritical fluids, existing below these thresholds, have limited solubility and reduced solvent strength. Consequently, supercritical fluids like CO2 demonstrate effective extraction capabilities for non-polar and moderately polar compounds, surpassing traditional subcritical solvent performance.
Phase Diagrams and Critical Points
Supercritical fluids exist above a substance's critical temperature and pressure, where distinct liquid and gas phases do not occur, whereas subcritical fluids exist below these critical parameters, maintaining separate phases. Phase diagrams highlight the critical point as the threshold at which the liquid-gas boundary ceases, marking the transition from subcritical to supercritical states. The unique density and solvent properties of supercritical fluids near the critical point enable applications such as extraction and material synthesis, distinct from subcritical fluid behavior.
Applications in Chemical Engineering Processes
Supercritical fluids, characterized by properties between liquids and gases, are widely used in chemical engineering for extraction, reaction media, and particle formation due to their tunable solvating power and enhanced mass transfer rates. Subcritical fluids, operating below critical temperature and pressure, find applications in hydrothermal processing, dyeing, and food sterilization, leveraging their unique solvency and thermal properties under moderate conditions. The choice between supercritical and subcritical fluids hinges on specific process requirements such as selectivity, diffusivity, and environmental impact in chemical manufacturing and material processing.
Extraction Efficiency: Supercritical vs Subcritical Methods
Supercritical fluids exhibit higher extraction efficiency compared to subcritical fluids due to their enhanced diffusivity and solvent power, enabling rapid penetration and solubilization of target compounds. The tunable density and viscosity of supercritical fluids, particularly supercritical CO2, facilitate selective extraction and improved mass transfer rates not achievable under subcritical conditions. Subcritical fluids typically require longer extraction times and higher solvent volumes, resulting in lower yield and selectivity when extracting bioactive compounds or essential oils.
Environmental and Safety Considerations
Supercritical fluids, such as supercritical CO2, offer environmentally friendly solvent properties with low toxicity and minimal waste production compared to traditional subcritical solvents, reducing hazardous chemical disposal and environmental contamination risks. Subcritical fluids often require higher volumes of volatile organic compounds (VOCs), increasing air pollution and the potential for flammability hazards, while supercritical fluids operate at controlled pressures and temperatures that enhance process safety and minimize explosion risks. Both fluids necessitate careful handling, but supercritical fluid systems typically incorporate advanced pressure control and monitoring technologies to mitigate risks associated with high-pressure operations.
Economic and Industrial Implications
Supercritical fluids, with their unique solvating power and tunable density, enable more efficient extraction and separation processes, reducing energy consumption and operational costs in industries such as pharmaceuticals, food, and petrochemicals. Subcritical fluids, operating under lower pressure and temperature conditions, offer simpler equipment requirements and reduced safety risks, making them economically attractive for large-scale industrial applications with less complex separations. The choice between supercritical and subcritical fluids impacts capital investment, process scalability, and product quality, directly influencing overall industrial profitability and sustainability.
Future Trends in Fluid Technology
Supercritical fluids, characterized by their unique properties above critical temperature and pressure, enable enhanced extraction and material processing applications, showing significant potential in sustainable technologies. Subcritical fluids, operating below critical points, continue to be optimized for targeted separation and environmental remediation with lower energy requirements. Future trends emphasize hybrid systems integrating supercritical and subcritical phases, advanced tunable solvent systems, and AI-driven process optimization to achieve higher efficiency and reduced environmental impact in fluid technology.
Critical point
Supercritical fluids exist above the critical point where distinct liquid and gas phases vanish, while subcritical fluids remain below this threshold, maintaining separate liquid and gas states.
Phase equilibrium
Supercritical fluids exhibit unique phase equilibrium properties characterized by a single homogeneous phase above critical temperature and pressure, unlike subcritical fluids which maintain distinct liquid and vapor phases separated by a coexistence curve.
Solvent power
Supercritical fluids exhibit enhanced solvent power compared to subcritical fluids due to their higher diffusivity and tunable density above the critical point.
Density fluctuation
Supercritical fluids exhibit significantly higher density fluctuations near the critical point compared to subcritical fluids, enhancing their solvating power and transport properties.
Isothermal compressibility
Supercritical fluids exhibit significantly lower isothermal compressibility compared to subcritical fluids due to their decreased density fluctuations and enhanced molecular interactions at temperatures and pressures above the critical point.
Supercritical extraction
Supercritical extraction utilizes supercritical fluids like CO2 above their critical temperature and pressure, enabling efficient, selective, and environmentally friendly separation of compounds compared to subcritical fluids.
Subcritical water hydrolysis
Subcritical water hydrolysis utilizes water at temperatures between 100degC and 374degC under high pressure to enhance hydrolytic reactions, offering an energy-efficient and environmentally friendly alternative to supercritical fluids in biomass conversion.
Near-critical region
Near-critical region fluid properties exhibit significant density fluctuations and enhanced solvation power, with supercritical fluids showing continuous phase transitions above critical temperature and pressure, whereas subcritical fluids remain below these points, maintaining distinct liquid and gas phases.
Dielectric constant variation
Supercritical fluids exhibit a dramatic decrease in dielectric constant compared to subcritical fluids, enabling enhanced solvent power and tunable polarity for diverse chemical processes.
Fluid tunability
Supercritical fluids offer superior tunability in density, viscosity, and solvating power compared to subcritical fluids, enabling more precise control in extraction and reaction processes.
Supercritical fluids vs Subcritical fluids Infographic
 njnir.com
njnir.com