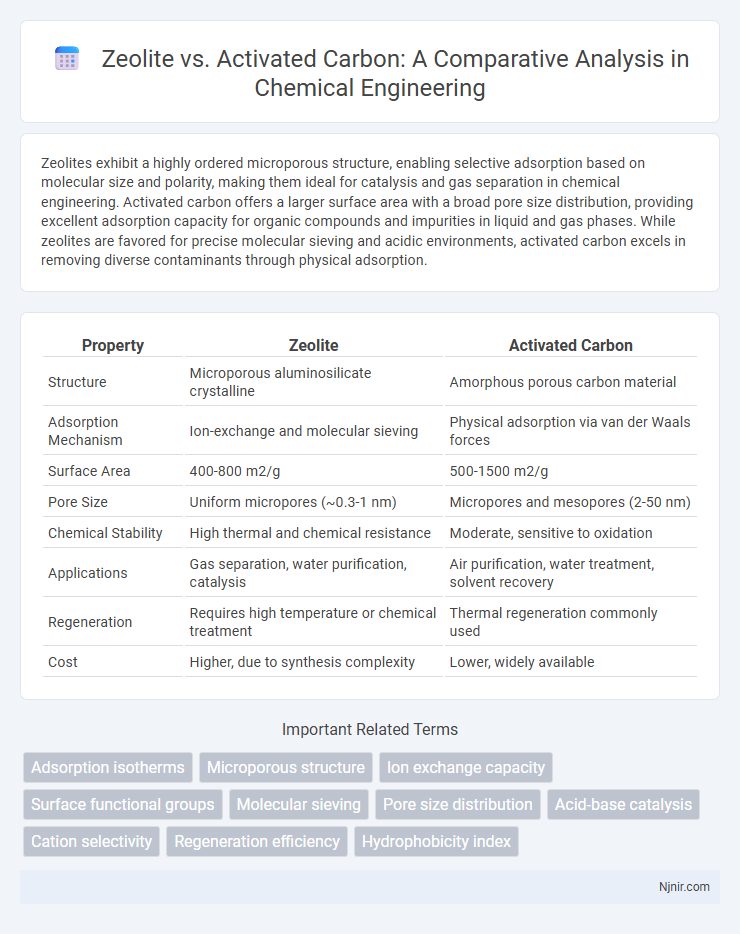
Zeolites exhibit a highly ordered microporous structure, enabling selective adsorption based on molecular size and polarity, making them ideal for catalysis and gas separation in chemical engineering. Activated carbon offers a larger surface area with a broad pore size distribution, providing excellent adsorption capacity for organic compounds and impurities in liquid and gas phases. While zeolites are favored for precise molecular sieving and acidic environments, activated carbon excels in removing diverse contaminants through physical adsorption.
Table of Comparison
| Property | Zeolite | Activated Carbon |
|---|---|---|
| Structure | Microporous aluminosilicate crystalline | Amorphous porous carbon material |
| Adsorption Mechanism | Ion-exchange and molecular sieving | Physical adsorption via van der Waals forces |
| Surface Area | 400-800 m2/g | 500-1500 m2/g |
| Pore Size | Uniform micropores (~0.3-1 nm) | Micropores and mesopores (2-50 nm) |
| Chemical Stability | High thermal and chemical resistance | Moderate, sensitive to oxidation |
| Applications | Gas separation, water purification, catalysis | Air purification, water treatment, solvent recovery |
| Regeneration | Requires high temperature or chemical treatment | Thermal regeneration commonly used |
| Cost | Higher, due to synthesis complexity | Lower, widely available |
Introduction to Zeolite and Activated Carbon
Zeolite is a crystalline aluminosilicate mineral known for its porous structure and high ion-exchange capacity, making it effective for gas separation, water purification, and catalysis. Activated carbon, derived from carbon-rich materials like coal or coconut shells, features a highly porous surface area ideal for adsorbing organic compounds, toxins, and impurities in air and water treatment. Both materials are widely utilized for environmental remediation and industrial processes due to their distinct adsorption and filtration properties.
Structural Differences Between Zeolite and Activated Carbon
Zeolite features a crystalline aluminosilicate framework with uniform micropores ranging from 0.3 to 1 nanometer, enabling selective molecular adsorption based on size and polarity. Activated carbon consists of a highly porous, amorphous carbon structure with a wide distribution of pore sizes, including micropores, mesopores, and macropores, which provides extensive surface area for adsorption of diverse organic molecules. The rigid, well-defined pore channels of zeolite contrast with the irregular, heterogeneous pore system of activated carbon, influencing their specific adsorption capacities and applications.
Mechanisms of Adsorption: Zeolite vs Activated Carbon
Zeolite adsorbs molecules primarily through ion-exchange and molecular sieving, leveraging its crystalline aluminosilicate framework with uniform micropores tailored for specific ion sizes. Activated carbon relies on physical adsorption driven by its extensive porous structure and large surface area, capturing a wide range of organic compounds via Van der Waals forces and hydrophobic interactions. While zeolite offers selective adsorption based on molecular size and charge, activated carbon provides broad-spectrum adsorption suitable for diverse contaminants.
Applications in Chemical Engineering Processes
Zeolite's highly porous crystalline structure and ion-exchange capabilities make it ideal for catalysis in petrochemical cracking, gas separation, and wastewater treatment. Activated carbon excels in adsorption processes due to its extensive surface area and microporosity, effectively removing organic contaminants, volatile compounds, and impurities in air and water purification. Both materials are integral in refining, environmental remediation, and gas purification, with zeolites preferred for selective molecular sieving and activated carbon for broad-spectrum adsorption.
Performance in Gas Separation and Purification
Zeolite exhibits superior selectivity in gas separation due to its uniform microporous structure and strong adsorption affinity for specific gas molecules, making it highly effective in removing moisture, CO2, and hydrocarbons from gas streams. Activated carbon offers a broader adsorption spectrum with high surface area and pore volume, excelling in trapping volatile organic compounds (VOCs) and non-polar gases but with less precise molecular discrimination compared to zeolite. Performance in gas purification depends on the target gases; zeolite is favored for selective separations in natural gas processing and air enrichment, while activated carbon is preferred for VOC removal and odor control.
Efficiency in Water and Wastewater Treatment
Zeolite exhibits high ion-exchange capacity and selective removal of heavy metals and ammonia, making it highly efficient in water and wastewater treatment. Activated carbon demonstrates superior adsorption of organic contaminants, chlorine, and odors, offering effective purification for a broad range of pollutants. Combining zeolite and activated carbon can enhance overall treatment efficiency by targeting diverse contaminants simultaneously.
Regeneration and Reusability Comparison
Zeolite and activated carbon differ significantly in regeneration and reusability; zeolite can be regenerated through thermal treatment or chemical washing with minimal loss of adsorption capacity, making it highly reusable for multiple cycles. Activated carbon often requires high-temperature steam or chemical regeneration, which can degrade its porous structure and reduce adsorption efficiency over time. Zeolite's crystalline framework offers greater stability during regeneration processes, enhancing longevity compared to the amorphous structure of activated carbon.
Environmental Impact and Sustainability
Zeolite offers superior environmental sustainability due to its natural abundance, biodegradability, and ability to be regenerated multiple times without significant loss in adsorption capacity, reducing waste and the need for frequent replacement. Activated carbon, while highly effective in pollutant removal, often relies on non-renewable sources like coal or wood and requires energy-intensive production processes, contributing to higher carbon emissions. The ecological footprint of zeolite is generally lower, making it a more sustainable choice for long-term environmental remediation and waste management applications.
Cost Analysis and Economic Considerations
Zeolite generally offers a lower initial cost and longer lifespan compared to activated carbon, making it a cost-effective choice for large-scale industrial applications. Activated carbon requires frequent replacement due to its saturation and degradation, increasing operational expenses over time. Economic considerations should include lifecycle costs, regeneration feasibility, and application-specific adsorption efficiency for accurate cost-benefit analysis.
Future Trends in Adsorbent Technologies
Zeolite and activated carbon both remain critical in adsorption technology, but future trends emphasize enhancing their selectivity and adsorption capacity through nanostructuring and functionalization. Research is advancing toward hybrid materials combining zeolites' molecular sieving with activated carbon's high surface area for improved contaminant removal and energy efficiency. Innovations in green synthesis methods and regeneration techniques are driving sustainability in adsorbent applications across water treatment, air purification, and industrial gas separation.
Adsorption isotherms
Zeolite exhibits higher selectivity and capacity in adsorption isotherms for polar molecules compared to activated carbon, which demonstrates greater adsorption for nonpolar organic compounds due to its larger surface area and pore volume.
Microporous structure
Zeolite's highly uniform microporous structure enables selective molecular sieving, whereas activated carbon features a more heterogeneous microporosity that favors adsorption of a broader range of molecules.
Ion exchange capacity
Zeolite exhibits a higher ion exchange capacity than activated carbon due to its crystalline aluminosilicate structure with uniform pore sizes that selectively trap ions.
Surface functional groups
Zeolite features surface functional groups primarily composed of oxygen atoms within its aluminosilicate framework, enabling ion-exchange and selective adsorption, whereas activated carbon possesses diverse surface functional groups such as carboxyl, hydroxyl, and carbonyl, which enhance its adsorption capacity and chemical reactivity.
Molecular sieving
Zeolite exhibits superior molecular sieving capabilities compared to activated carbon due to its uniform microporous structure and precise pore size distribution that selectively adsorb molecules based on size and shape.
Pore size distribution
Zeolite exhibits a uniform microporous structure with pore sizes typically below 2 nanometers, while activated carbon features a broader pore size distribution ranging from micropores (less than 2 nm) to mesopores (2-50 nm), enhancing its versatility in adsorption applications.
Acid-base catalysis
Zeolite exhibits superior acid-base catalysis compared to activated carbon due to its well-defined microporous structure and strong Bronsted and Lewis acid sites.
Cation selectivity
Zeolites exhibit higher cation selectivity than activated carbon due to their crystalline aluminosilicate framework with negatively charged sites that preferentially adsorb specific cations based on size and charge.
Regeneration efficiency
Zeolite regeneration efficiency surpasses activated carbon by maintaining higher adsorption capacity and structural integrity over multiple cycles, enabling cost-effective and sustainable reuse.
Hydrophobicity index
Zeolite exhibits a lower hydrophobicity index compared to activated carbon, making activated carbon more effective for adsorbing non-polar, hydrophobic compounds in water treatment processes.
zeolite vs activated carbon Infographic
 njnir.com
njnir.com