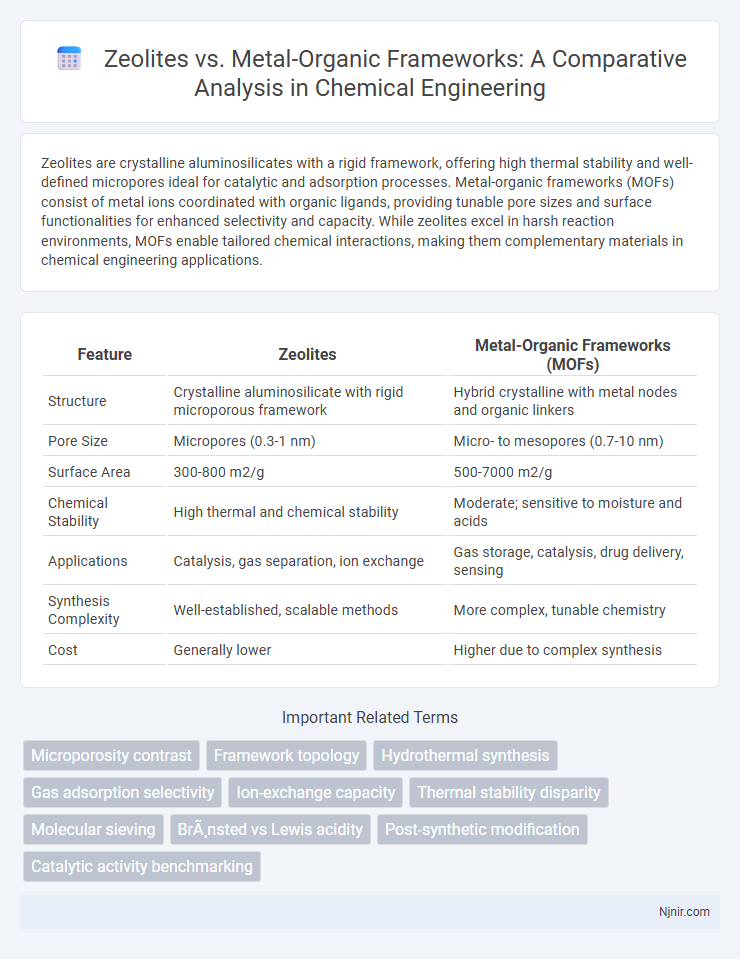
Zeolites are crystalline aluminosilicates with a rigid framework, offering high thermal stability and well-defined micropores ideal for catalytic and adsorption processes. Metal-organic frameworks (MOFs) consist of metal ions coordinated with organic ligands, providing tunable pore sizes and surface functionalities for enhanced selectivity and capacity. While zeolites excel in harsh reaction environments, MOFs enable tailored chemical interactions, making them complementary materials in chemical engineering applications.
Table of Comparison
| Feature | Zeolites | Metal-Organic Frameworks (MOFs) |
|---|---|---|
| Structure | Crystalline aluminosilicate with rigid microporous framework | Hybrid crystalline with metal nodes and organic linkers |
| Pore Size | Micropores (0.3-1 nm) | Micro- to mesopores (0.7-10 nm) |
| Surface Area | 300-800 m2/g | 500-7000 m2/g |
| Chemical Stability | High thermal and chemical stability | Moderate; sensitive to moisture and acids |
| Applications | Catalysis, gas separation, ion exchange | Gas storage, catalysis, drug delivery, sensing |
| Synthesis Complexity | Well-established, scalable methods | More complex, tunable chemistry |
| Cost | Generally lower | Higher due to complex synthesis |
Introduction to Porous Materials in Chemical Engineering
Zeolites and metal-organic frameworks (MOFs) are essential porous materials widely used in chemical engineering for catalysis, gas separation, and adsorption due to their high surface area and tunable pore structures. Zeolites, crystalline aluminosilicates with uniform micropores, offer exceptional thermal stability and ion-exchange capacities, making them ideal for industrial catalytic processes. MOFs consist of metal ions coordinated to organic ligands, providing greater structural diversity and adjustable pore sizes, enabling tailored applications in gas storage and molecular sieving.
Structural Overview: Zeolites vs Metal-Organic Frameworks
Zeolites are crystalline aluminosilicate minerals with a microporous, three-dimensional framework of SiO4 and AlO4 tetrahedra, featuring uniform pore sizes typically between 3 to 10 A. Metal-Organic Frameworks (MOFs) consist of metal ion nodes connected by organic ligands, forming highly tunable, porous structures with surface areas often exceeding 7000 m2/g and pore sizes ranging from microporous to mesoporous scales. The rigid, well-defined channels in zeolites contrast with the versatile, modular architecture of MOFs, enabling customized porosity and functionalization for targeted applications.
Synthesis Methods and Scalability
Zeolites are synthesized primarily through hydrothermal or solvothermal methods, exhibiting well-established, cost-effective scalability for industrial applications due to their crystalline aluminosilicate framework. Metal-organic frameworks (MOFs) require more complex synthesis techniques such as solvothermal, microwave-assisted, or mechanochemical methods, often limiting large-scale production because of high costs and sensitivity to reaction conditions. Advances in continuous flow reactors and green synthesis are improving MOF scalability, yet zeolites remain superior for high-volume manufacturing in catalysis and adsorption.
Surface Area and Porosity Comparison
Zeolites exhibit high thermal stability with microporous structures typically featuring surface areas around 300-600 m2/g, while metal-organic frameworks (MOFs) demonstrate exceptionally high surface areas often exceeding 4,000 m2/g due to their tunable porosity and larger pore volumes. MOFs offer greater structural diversity and adjustable pore sizes ranging from microporous to mesoporous, enabling enhanced gas storage and catalysis applications compared to the predominantly microporous and rigid framework of zeolites. The enhanced porosity and surface area of MOFs facilitate higher adsorption capacities, making them superior in applications requiring molecular sieving and gas separation.
Thermal and Chemical Stability
Zeolites exhibit superior thermal stability withstanding temperatures up to 1000degC, while metal-organic frameworks (MOFs) typically degrade above 300-400degC due to their organic linkers. Chemically, zeolites maintain robustness in acidic and basic environments, attributed to their rigid aluminosilicate frameworks, whereas MOFs often suffer from hydrolysis and chemical breakdown in moisture or harsh chemicals. The intrinsic inorganic nature of zeolites ensures long-term stability in industrial catalytic and adsorption processes, contrasting with the tunable but less chemically resilient MOFs.
Tunability and Functionalization
Zeolites possess a rigid, crystalline aluminosilicate framework with limited tunability primarily achieved through ion exchange and isomorphic substitution, making their functionalization constrained by pore size and structure. Metal-organic frameworks (MOFs) exhibit exceptional tunability due to their modular construction from metal nodes and organic linkers, allowing precise control over pore size, shape, and chemical functionality. Functionalization of MOFs is highly versatile, including post-synthetic modification and linker variation, enabling tailored properties for gas storage, catalysis, and sensing applications.
Applications in Catalysis
Zeolites exhibit exceptional catalytic properties in petrochemical refining and hydrocarbon cracking due to their well-defined microporous structure and strong acidity. Metal-organic frameworks (MOFs) offer tunable pore sizes and diverse active sites, making them highly effective in selective catalysis and gas-phase reactions such as CO2 conversion and methane activation. The combination of zeolites' thermal stability and MOFs' structural versatility expands catalytic applications across energy conversion and environmental remediation.
Gas Separation and Storage Capabilities
Zeolites exhibit high thermal stability and molecular sieving properties, making them effective for gas separation by selectively adsorbing molecules based on size and polarity. Metal-organic frameworks (MOFs) offer unparalleled surface areas and tunable pore structures, enhancing gas storage capacities for hydrogen, methane, and carbon dioxide. MOFs outperform zeolites in gas uptake due to their customizable organic linkers, although zeolites remain preferred for industrial applications requiring robustness and cost efficiency.
Environmental and Economic Considerations
Zeolites offer high thermal stability and are commercially established for gas separation and catalysis, with relatively low production costs and proven recyclability that favor environmental sustainability. Metal-organic frameworks (MOFs) provide tunable pore structures and exceptional surface areas, enabling superior selectivity and storage capacities but often require expensive synthesis and exhibit lower stability under harsh conditions, impacting their economic viability. Both materials contribute to reducing environmental footprints through energy-efficient processes, yet zeolites currently dominate industrial applications due to cost-effectiveness and durability, while MOFs continue to advance for niche, high-performance uses.
Future Directions and Emerging Trends
Zeolites and metal-organic frameworks (MOFs) are advancing toward enhanced stability and selectivity for catalysis and gas separation, with research emphasizing tunable pore structures and hybrid composites. Emerging trends in MOFs include integrating stimuli-responsive functionalities and scalable synthesis methods to overcome current limitations in industrial applications. Future directions highlight the development of zeolite-MOF composites to combine zeolites' thermal robustness with MOFs' structural versatility for superior performance in environmental and energy sectors.
Microporosity contrast
Zeolites exhibit rigid, crystalline microporosity with uniform pore sizes typically below 1 nm, whereas metal-organic frameworks feature tunable, often larger microporous structures ranging from 0.5 to 2 nm due to their customizable organic linkers.
Framework topology
Zeolites exhibit rigid, well-defined crystalline framework topology based on aluminosilicate tetrahedra, while metal-organic frameworks (MOFs) offer highly tunable and diverse topology through customizable metal nodes and organic linkers.
Hydrothermal synthesis
Hydrothermal synthesis enables precise control over the crystallinity and pore structure of zeolites and metal-organic frameworks, with zeolites favoring aluminosilicate frameworks under high-temperature aqueous conditions and metal-organic frameworks requiring milder, metal-ligand coordination environments.
Gas adsorption selectivity
Zeolites exhibit high gas adsorption selectivity through uniform microporous structures and strong ion-exchange sites, while metal-organic frameworks (MOFs) offer tunable pore sizes and functional groups enabling customizable and enhanced selectivity for targeted gas molecules.
Ion-exchange capacity
Zeolites exhibit high ion-exchange capacity due to their crystalline aluminosilicate framework with uniform pore sizes, while metal-organic frameworks offer tunable pore structures and functional groups that can enhance ion-exchange selectivity and capacity depending on their metal nodes and organic linkers.
Thermal stability disparity
Zeolites exhibit superior thermal stability up to 1000degC due to their robust aluminosilicate framework, whereas metal-organic frameworks (MOFs) typically degrade above 300-400degC because of their organic linker components.
Molecular sieving
Zeolites exhibit highly selective molecular sieving due to their uniform microporous aluminosilicate frameworks, whereas metal-organic frameworks offer tunable pore sizes and functional groups enabling customizable molecular sieving for targeted separation processes.
Brønsted vs Lewis acidity
Zeolites primarily exhibit Bronsted acidity through their hydroxyl groups facilitating proton donation, whereas metal-organic frameworks predominantly display Lewis acidity via coordinatively unsaturated metal sites acting as electron pair acceptors.
Post-synthetic modification
Post-synthetic modification of metal-organic frameworks enables tailored functionalization and enhanced performance distinct from the more rigid and structurally constrained zeolites.
Catalytic activity benchmarking
Zeolites exhibit superior catalytic activity in acid-catalyzed reactions due to their well-defined microporous structures, while metal-organic frameworks offer tunable active sites and higher surface areas, enabling enhanced catalytic performance in selective oxidation and gas-phase reactions.
Zeolites vs Metal-organic frameworks Infographic
 njnir.com
njnir.com