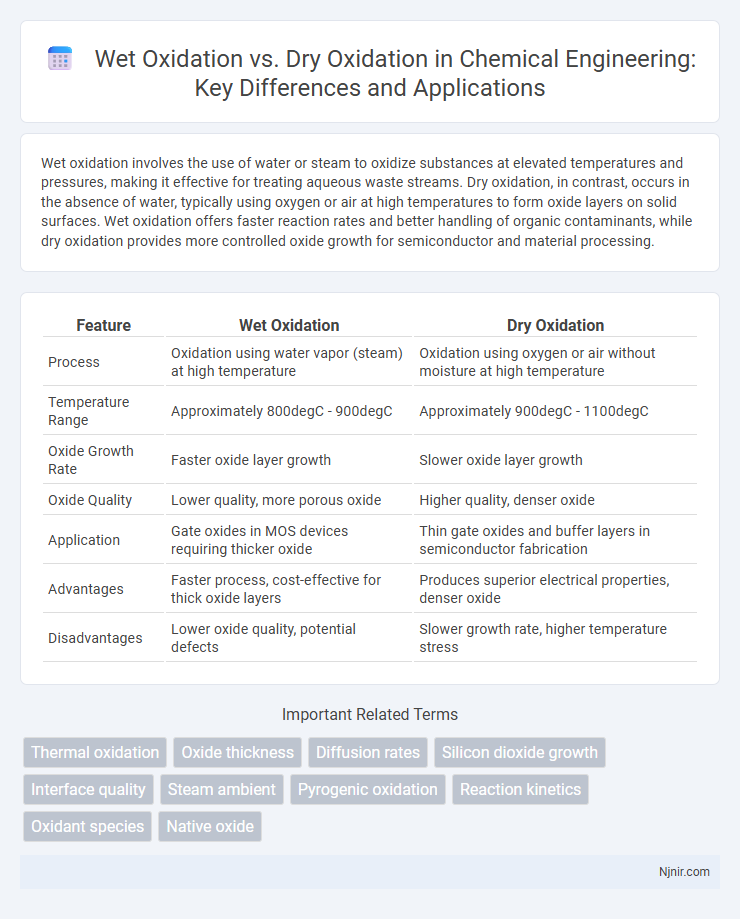
Wet oxidation involves the use of water or steam to oxidize substances at elevated temperatures and pressures, making it effective for treating aqueous waste streams. Dry oxidation, in contrast, occurs in the absence of water, typically using oxygen or air at high temperatures to form oxide layers on solid surfaces. Wet oxidation offers faster reaction rates and better handling of organic contaminants, while dry oxidation provides more controlled oxide growth for semiconductor and material processing.
Table of Comparison
| Feature | Wet Oxidation | Dry Oxidation |
|---|---|---|
| Process | Oxidation using water vapor (steam) at high temperature | Oxidation using oxygen or air without moisture at high temperature |
| Temperature Range | Approximately 800degC - 900degC | Approximately 900degC - 1100degC |
| Oxide Growth Rate | Faster oxide layer growth | Slower oxide layer growth |
| Oxide Quality | Lower quality, more porous oxide | Higher quality, denser oxide |
| Application | Gate oxides in MOS devices requiring thicker oxide | Thin gate oxides and buffer layers in semiconductor fabrication |
| Advantages | Faster process, cost-effective for thick oxide layers | Produces superior electrical properties, denser oxide |
| Disadvantages | Lower oxide quality, potential defects | Slower growth rate, higher temperature stress |
Overview of Oxidation Processes in Chemical Engineering
Wet oxidation involves reacting organic and inorganic substances with oxygen in the presence of water at elevated temperatures and pressures, enhancing oxidation rates and allowing efficient treatment of wastewater and sludge. Dry oxidation occurs without water, relying on oxygen gas at high temperatures to form thin oxide layers on metal surfaces, commonly used in semiconductor fabrication for silicon wafer oxidation. Both processes are fundamental in chemical engineering for controlling reaction environments, optimizing oxidation efficiency, and influencing material properties in industrial applications.
Fundamentals of Wet Oxidation
Wet oxidation involves the chemical reaction of organic or inorganic substances with oxygen in the presence of liquid water at elevated temperatures and pressures, facilitating faster reaction rates compared to dry oxidation. The presence of water as a medium enhances the diffusion of oxygen and provides a solvent environment that promotes the breakdown of complex compounds into simpler molecules. This process is widely used for waste treatment, organic compound degradation, and chemical synthesis due to its efficiency and ability to operate under milder conditions than dry oxidation.
Principles of Dry Oxidation
Dry oxidation involves the growth of a silicon dioxide (SiO2) layer on a silicon wafer by exposing it to pure oxygen (O2) at high temperatures, typically between 900degC and 1200degC. The fundamental principle is the diffusion of oxygen molecules through the existing oxide layer to react with silicon atoms, producing a highly dense and uniform oxide film with superior electrical properties compared to wet oxidation. This process results in slower oxide growth rates but yields better quality oxides critical for gate dielectrics in MOSFET devices.
Chemical Mechanisms: Wet vs Dry Oxidation
Wet oxidation employs water vapor at elevated temperatures to enhance the reaction rate by generating hydroxyl radicals that aggressively react with silicon surfaces, forming a thicker silicon dioxide layer more rapidly. Dry oxidation uses pure oxygen gas, creating a denser and higher quality oxide layer through direct oxygen diffusion and silicon oxidation without the involvement of water-derived radicals. The chemical mechanism of wet oxidation favors faster growth due to the higher diffusivity of water molecules and more reactive species, while dry oxidation provides superior oxide uniformity and electrical properties essential for semiconductor device fabrication.
Key Applications in Industry
Wet oxidation is widely applied in wastewater treatment industries for the efficient decomposition of organic pollutants and hazardous wastes due to its ability to operate at lower temperatures and pressures. Dry oxidation finds extensive use in the semiconductor manufacturing sector for the growth of high-quality silicon dioxide layers on silicon wafers, essential for device insulation and gate dielectrics. Industries such as chemical processing leverage wet oxidation for the oxidation of hazardous organic compounds, while dry oxidation is preferred in microelectronics for precise thermal oxidation processes.
Process Conditions and Equipment Requirements
Wet oxidation operates at lower temperatures between 250degC and 300degC and pressures around 1 MPa, requiring reactors resistant to steam and acidic conditions, such as stainless steel autoclaves. Dry oxidation proceeds at higher temperatures ranging from 900degC to 1200degC in atmospheric oxygen, necessitating high-temperature oxidation furnaces capable of precise temperature control and diffusion barrier layers to maintain silicon wafer integrity. Wet oxidation yields faster oxide growth rates due to enhanced diffusion of oxidizing species in steam, while dry oxidation produces higher quality, denser silicon dioxide layers ideal for microelectronic device fabrication.
Efficiency and Yield Comparisons
Wet oxidation offers higher growth rates and improved oxide quality due to enhanced diffusion of oxidizing species, resulting in more efficient processes for thicker oxide layers. Dry oxidation provides superior oxide purity and electrical characteristics, yielding better interface quality and lower defect densities essential for high-performance semiconductor devices. Efficiency in wet oxidation favors rapid oxide formation, while dry oxidation excels in producing precise, high-yield thin oxides for advanced microelectronics.
Environmental Impacts and Byproducts
Wet oxidation produces fewer hazardous byproducts compared to dry oxidation due to its use of water vapor, which results in lower emissions of nitrogen oxides and reduces particulate matter formation. Dry oxidation typically generates higher levels of nitrogen oxides and silicon dioxide particulates, contributing to air pollution and requiring more extensive emission controls. Environmental impacts of wet oxidation are generally less severe, with reduced acid rain potential and lower toxicity in waste streams, promoting greener semiconductor manufacturing processes.
Advantages and Limitations of Each Method
Wet oxidation enables faster oxide growth rates and improved step coverage, making it ideal for thick oxide layers and reducing processing time. However, it produces lower-quality oxides with higher impurity levels and inferior electrical properties compared to dry oxidation. Dry oxidation creates high-quality, dense oxide layers with superior dielectric strength and interface quality, but requires longer processing times and is less efficient for thick oxide formation.
Selection Criteria for Industrial Implementation
Wet oxidation offers faster oxide growth rates and lower thermal budgets, making it suitable for processes requiring rapid oxide formation and reduced thermal stress. Dry oxidation provides superior oxide quality, with higher density and better electrical properties, ideal for applications demanding high-quality gate oxides in semiconductor manufacturing. Selection criteria for industrial implementation depend on balancing process speed, oxide integrity, thermal budget constraints, and specific device performance requirements.
Thermal oxidation
Wet oxidation employs steam to grow silicon dioxide layers faster at lower temperatures, while dry oxidation uses pure oxygen to produce thinner, denser oxide layers with superior electrical quality in thermal oxidation processes.
Oxide thickness
Wet oxidation produces thicker silicon dioxide layers faster than dry oxidation, which yields thinner, higher-quality oxide films ideal for gate oxides in semiconductor devices.
Diffusion rates
Wet oxidation offers significantly higher diffusion rates due to the presence of steam enhancing oxide growth, whereas dry oxidation results in slower diffusion rates and thinner oxide layers because it relies on oxygen molecules alone.
Silicon dioxide growth
Wet oxidation grows silicon dioxide faster with higher oxide quality due to steam-enhanced reaction rates, while dry oxidation yields thinner, denser oxides with superior electrical properties but slower growth.
Interface quality
Wet oxidation produces a thicker oxide layer with faster growth but results in poorer interface quality due to higher interface trap densities, whereas dry oxidation yields a thinner, denser oxide with superior interface quality and lower defect densities.
Steam ambient
Steam ambient wet oxidation enhances silicon dioxide growth rate compared to dry oxidation by increasing diffusion of oxidizing species and reducing oxide defects.
Pyrogenic oxidation
Pyrogenic oxidation involves dry oxidation processes where silicon wafers are exposed to oxygen at high temperatures to grow thin, high-quality silicon dioxide layers, whereas wet oxidation uses steam to accelerate oxide growth but can introduce more defects.
Reaction kinetics
Wet oxidation exhibits faster reaction kinetics than dry oxidation due to the higher diffusion rates of water vapor, enabling more rapid silicon dioxide growth on semiconductor surfaces.
Oxidant species
Wet oxidation utilizes reactive hydroxyl radicals (*OH) generated from steam, while dry oxidation relies on molecular oxygen (O2) as the primary oxidant species.
Native oxide
Wet oxidation produces thicker, less dense native oxide layers with higher growth rates compared to the thinner, denser native oxides formed by dry oxidation.
Wet oxidation vs Dry oxidation Infographic
 njnir.com
njnir.com