Pyrolysis and gasification are thermochemical processes that convert organic materials into valuable products through heat treatment in controlled atmospheres. Pyrolysis occurs in the absence of oxygen, producing bio-oil, char, and syngas, while gasification operates with limited oxygen, generating a high-energy syngas primarily composed of carbon monoxide, hydrogen, and methane. These differences influence their efficiency, product composition, and applications in sustainable energy and waste management.
Table of Comparison
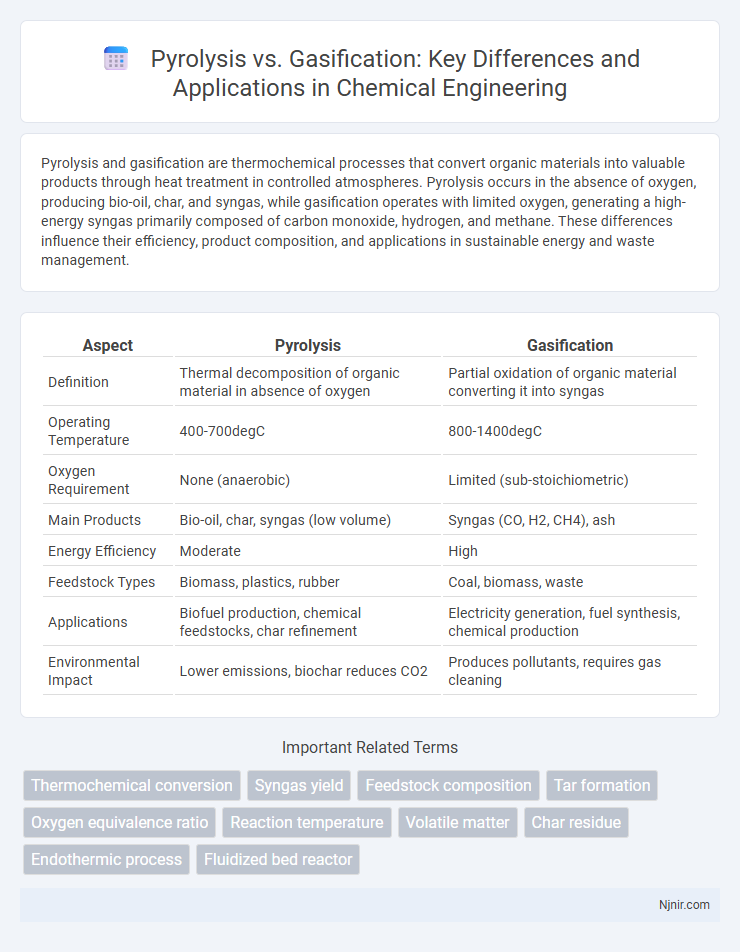
| Aspect | Pyrolysis | Gasification |
|---|---|---|
| Definition | Thermal decomposition of organic material in absence of oxygen | Partial oxidation of organic material converting it into syngas |
| Operating Temperature | 400-700degC | 800-1400degC |
| Oxygen Requirement | None (anaerobic) | Limited (sub-stoichiometric) |
| Main Products | Bio-oil, char, syngas (low volume) | Syngas (CO, H2, CH4), ash |
| Energy Efficiency | Moderate | High |
| Feedstock Types | Biomass, plastics, rubber | Coal, biomass, waste |
| Applications | Biofuel production, chemical feedstocks, char refinement | Electricity generation, fuel synthesis, chemical production |
| Environmental Impact | Lower emissions, biochar reduces CO2 | Produces pollutants, requires gas cleaning |
Introduction to Pyrolysis and Gasification
Pyrolysis and gasification are thermochemical processes that convert organic materials into valuable products through controlled heating. Pyrolysis decomposes biomass in an oxygen-free environment, producing char, bio-oil, and syngas, while gasification partially oxidizes feedstock to generate syngas rich in hydrogen and carbon monoxide. Both technologies play crucial roles in renewable energy production, waste management, and biofuel generation.
Fundamental Principles of Pyrolysis
Pyrolysis involves the thermal decomposition of organic material in the absence of oxygen, breaking down complex polymers into simpler molecules such as bio-oil, syngas, and char through heat-induced chemical reactions. The process typically occurs at temperatures between 300degC and 900degC, facilitating the conversion of biomass into valuable energy carriers without combustion. Pyrolysis differs from gasification by operating in an oxygen-free environment, emphasizing the fundamental principle of thermal cracking rather than partial oxidation.
Core Mechanisms of Gasification
Gasification converts carbonaceous materials into syngas through partial oxidation at high temperatures, typically between 700degC and 1600degC, involving reactions like pyrolysis, oxidation, and reduction. Core mechanisms include the controlled supply of oxygen or steam, which facilitates the conversion of solid fuel into a combustible gas mixture composed mainly of CO, H2, and CO2. This process differs from pyrolysis by integrating oxidation reactions that enhance energy recovery and reduce tar formation, making gasification more efficient for energy generation.
Feedstock Selection and Preparation
Feedstock selection for pyrolysis and gasification depends on moisture content, particle size, and composition; pyrolysis typically requires drier biomass with low moisture below 20%, while gasification tolerates moisture up to 30%. Preparation processes involve shredding or pelletizing to achieve uniform particle size, enhancing heat transfer and reaction efficiency in both technologies. Choosing feedstock with low ash content minimizes reactor fouling and extends operational life in pyrolysis and gasification systems.
Operating Conditions and Reactor Designs
Pyrolysis operates under oxygen-starved environments at temperatures typically between 300degC and 700degC, favoring thermal decomposition to produce bio-oil, char, and syngas, while gasification occurs in limited oxygen or steam presence at higher temperatures ranging from 700degC to 1500degC, focusing on syngas generation. Pyrolysis reactors commonly use fixed-bed, fluidized-bed, or rotary kiln designs that optimize heat transfer for solid biomass conversion, whereas gasification employs updraft, downdraft, or fluidized-bed reactors with intricate feedstock handling and gas-solid contact to enhance gas quality. Reactor design significantly impacts product distribution, with pyrolysis requiring rapid heating rates to maximize liquid yields and gasification depending on precise control of oxygen supply to maintain partial oxidation conditions.
Product Yields and Output Composition
Pyrolysis primarily produces bio-oil, syngas, and char, with yields heavily dependent on temperature and heating rate; slow pyrolysis favors char production, while fast pyrolysis maximizes liquid bio-oil output. Gasification generates a combustible syngas mainly composed of hydrogen, carbon monoxide, carbon dioxide, and methane, with minimal tar and char residues due to higher operational temperatures exceeding 700degC. Product composition in pyrolysis offers higher liquid fuel potential, whereas gasification provides a versatile gaseous fuel stream suitable for power and chemical synthesis.
Energy Efficiency and Process Integration
Pyrolysis achieves moderate energy efficiency by thermally decomposing organic materials in an oxygen-free environment, producing bio-oil, syngas, and char with lower energy input compared to gasification. Gasification operates at higher temperatures with controlled oxygen supply, converting feedstock into a more energy-dense syngas, yielding superior overall energy efficiency for power generation and chemical synthesis. Process integration in pyrolysis often involves downstream upgrading of bio-oil and char utilization, whereas gasification systems benefit from seamless integration with combined heat and power (CHP) plants, enhancing total energy recovery and operational efficiency.
Environmental Impacts and Emissions
Pyrolysis produces bio-oil, syngas, and char through thermal decomposition in the absence of oxygen, resulting in lower emissions of NOx and SOx compared to conventional combustion. Gasification partially oxidizes biomass or waste at high temperatures, generating syngas with a higher hydrogen and carbon monoxide content, but it can emit higher levels of tars and particulate matter if not properly managed. Both processes offer cleaner alternatives to fossil fuels by reducing greenhouse gas emissions, though gasification typically requires advanced gas cleaning systems to minimize environmental pollutants effectively.
Industrial Applications and Case Studies
Pyrolysis and gasification are advanced thermal conversion technologies extensively applied in industrial waste management and energy production. Pyrolysis efficiently processes biomass and plastic waste into bio-oil and char, demonstrated by companies like Agilyx converting mixed plastics into synthetic fuels, while gasification is favored for syngas production from coal and municipal solid waste, evidenced by the Shell Rhine Gasification Plant powering chemical synthesis. Industrial case studies reveal pyrolysis excels in producing liquid fuels for decentralized energy, whereas gasification supports large-scale integrated energy systems and hydrogen generation, optimizing carbon utilization and reducing carbon emissions.
Future Trends and Technological Advances
Emerging trends in pyrolysis emphasize enhanced catalyst development and integration with biochar production to boost carbon sequestration and energy yield. Gasification technology advances prioritize plasma gasification and hybrid systems combining gasification with renewable energy sources for higher efficiency and reduced emissions. Both processes benefit from digitalization and AI-driven process optimization to maximize conversion rates and minimize environmental impact.
Thermochemical conversion
Pyrolysis thermochemically decomposes organic material in the absence of oxygen producing bio-oil, char, and syngas, while gasification partially oxidizes biomass at high temperatures to generate primarily syngas composed of hydrogen, carbon monoxide, and methane.
Syngas yield
Gasification produces a higher syngas yield with a richer hydrogen and carbon monoxide content compared to pyrolysis, which generates more bio-oil and char but less syngas.
Feedstock composition
Feedstock composition significantly influences pyrolysis and gasification processes, with pyrolysis favoring high-carbon, low-moisture materials and gasification accommodating a broader range of feedstocks including those with higher moisture and ash content.
Tar formation
Pyrolysis generates higher tar levels due to slow thermal decomposition of biomass, whereas gasification operates at higher temperatures that significantly reduce tar formation through partial oxidation processes.
Oxygen equivalence ratio
Pyrolysis operates at an oxygen equivalence ratio near zero, enabling thermal decomposition without combustion, whereas gasification uses a controlled oxygen equivalence ratio typically between 0.2 and 0.4 to partially oxidize biomass into syngas.
Reaction temperature
Pyrolysis typically occurs at lower reaction temperatures between 300-700degC, while gasification requires higher temperatures ranging from 700-1,200degC to convert organic materials into syngas.
Volatile matter
Gasification produces more volatile matter conversion than pyrolysis due to higher temperature and partial oxidation enhancing volatile release and syngas yield.
Char residue
Gasification produces significantly less char residue than pyrolysis by converting a higher percentage of carbon feedstock into syngas through partial oxidation.
Endothermic process
Pyrolysis and gasification are both endothermic thermochemical processes that decompose organic materials, with pyrolysis occurring in an oxygen-free environment to produce biochar, tar, and syngas, while gasification uses limited oxygen or steam to primarily generate syngas rich in carbon monoxide and hydrogen.
Fluidized bed reactor
Fluidized bed reactors enhance pyrolysis and gasification efficiency by providing uniform temperature distribution and improved gas-solid contact, optimizing biochar yield in pyrolysis and syngas quality in gasification.
Pyrolysis vs Gasification Infographic
 njnir.com
njnir.com