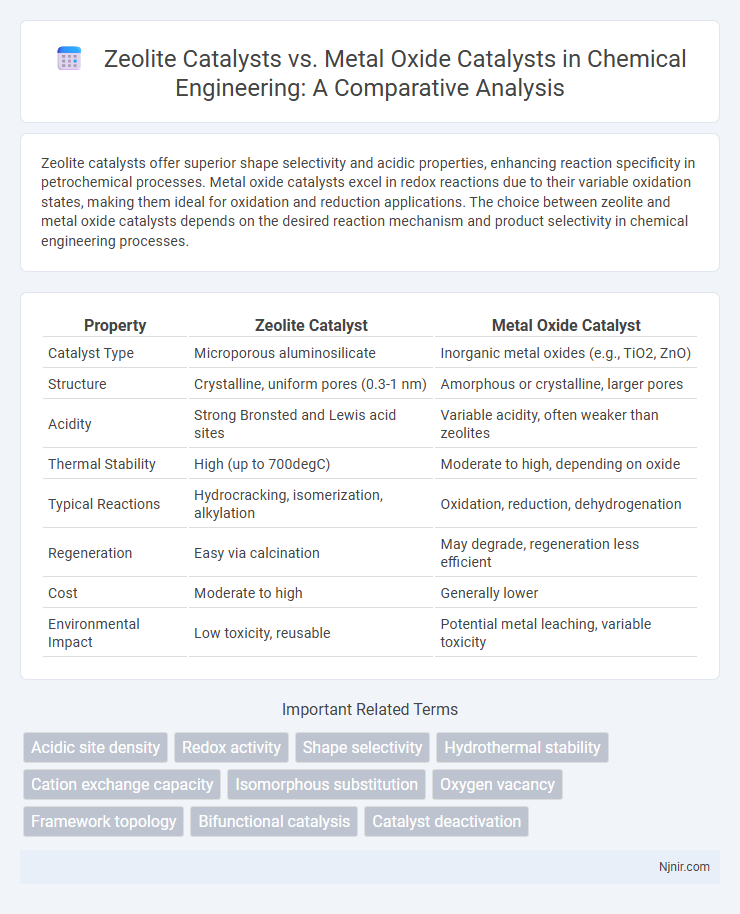
Zeolite catalysts offer superior shape selectivity and acidic properties, enhancing reaction specificity in petrochemical processes. Metal oxide catalysts excel in redox reactions due to their variable oxidation states, making them ideal for oxidation and reduction applications. The choice between zeolite and metal oxide catalysts depends on the desired reaction mechanism and product selectivity in chemical engineering processes.
Table of Comparison
| Property | Zeolite Catalyst | Metal Oxide Catalyst |
|---|---|---|
| Catalyst Type | Microporous aluminosilicate | Inorganic metal oxides (e.g., TiO2, ZnO) |
| Structure | Crystalline, uniform pores (0.3-1 nm) | Amorphous or crystalline, larger pores |
| Acidity | Strong Bronsted and Lewis acid sites | Variable acidity, often weaker than zeolites |
| Thermal Stability | High (up to 700degC) | Moderate to high, depending on oxide |
| Typical Reactions | Hydrocracking, isomerization, alkylation | Oxidation, reduction, dehydrogenation |
| Regeneration | Easy via calcination | May degrade, regeneration less efficient |
| Cost | Moderate to high | Generally lower |
| Environmental Impact | Low toxicity, reusable | Potential metal leaching, variable toxicity |
Introduction to Zeolite and Metal Oxide Catalysts
Zeolite catalysts, composed of crystalline aluminosilicates with a microporous structure, offer high surface area and shape-selective properties that enhance acid-catalyzed reactions. Metal oxide catalysts, such as titanium dioxide and vanadium oxide, provide redox activity and thermal stability crucial for oxidation and dehydrogenation processes. The distinct physicochemical characteristics and catalytic mechanisms of zeolites and metal oxides determine their application specificity in petrochemical, environmental, and fine chemical industries.
Structural Differences: Zeolites vs Metal Oxides
Zeolite catalysts possess a crystalline aluminosilicate framework with uniform microporous channels that enable shape-selective reactions, enhancing molecular sieving and adsorption properties. Metal oxide catalysts feature a less ordered, often amorphous or polycrystalline structure with variable oxidation states and surface defects that promote redox and acid-base reactions. The distinct pore architecture and acidity of zeolites contrast with the flexible surface chemistry and higher thermal stability of metal oxides, influencing their catalytic performance in industrial processes.
Surface Area and Porosity Comparison
Zeolite catalysts exhibit a highly ordered microporous structure with surface areas typically ranging from 300 to 800 m2/g, enabling selective adsorption and shape-selective catalysis. Metal oxide catalysts generally possess lower surface areas, often between 50 and 200 m2/g, with predominantly mesoporous or macroporous structures that favor bulk diffusion and active site accessibility. The distinct pore size distribution and higher surface area of zeolites enhance catalytic activity in reactions requiring molecular sieving, whereas metal oxides are more suited for oxidation and redox processes due to their variable porosity and surface characteristics.
Acidic and Basic Properties in Catalytic Processes
Zeolite catalysts exhibit strong Bronsted and Lewis acidic sites, enabling selective acid-catalyzed reactions such as hydrocarbon cracking and isomerization, while their microporous structure enhances shape selectivity. Metal oxide catalysts present versatile acidic and basic properties depending on their composition, with commonly used oxides like MgO offering basic sites for reactions like aldol condensation, and TiO2 providing Lewis acidic character for oxidation processes. The acidic strength and distribution in zeolites often lead to higher selectivity in acid-catalyzed reactions, whereas metal oxides provide tunable acidity and basicity for a broader range of catalytic applications.
Thermal Stability and Durability
Zeolite catalysts exhibit superior thermal stability due to their crystalline aluminosilicate framework, maintaining structural integrity at temperatures above 700degC, which enhances durability in high-temperature reactions. Metal oxide catalysts generally have lower thermal stability, often undergoing phase changes or sintering at elevated temperatures around 500-600degC, leading to reduced catalytic lifespan. The robust microporous structure of zeolites also contributes to prolonged durability by resisting deactivation from coking and metal sintering compared to metal oxides.
Selectivity and Reaction Mechanisms
Zeolite catalysts exhibit high selectivity due to their well-defined microporous structure and acidic sites, which enable shape-selective reactions and precise control over product distribution. Metal oxide catalysts provide versatile redox properties, facilitating diverse reaction pathways but often with broader product spectra and lower selectivity compared to zeolites. Reaction mechanisms in zeolite catalysts typically involve protonation and carbocation intermediates, whereas metal oxide catalysts utilize oxygen vacancies and electron transfer processes to activate reactants.
Industrial Applications: Zeolites vs. Metal Oxides
Zeolite catalysts are widely used in petroleum refining and petrochemical industries due to their high selectivity, shape selectivity, and strong acid sites, enabling efficient hydrocracking, fluid catalytic cracking (FCC), and isomerization processes. Metal oxide catalysts, such as titanium dioxide and zinc oxide, excel in oxidation, hydrogenation, and dehydrogenation reactions important in producing chemicals like methanol, ammonia, and synthesis gas. Industrial applications favor zeolites for processes requiring molecular sieving and acid catalysis, while metal oxides are preferred for redox reactions and catalytic converters in emission control.
Synthesis and Modification Techniques
Zeolite catalysts are synthesized through hydrothermal crystallization using sources like silica, alumina, and structure-directing agents, with modification techniques such as ion exchange, dealumination, and surface functionalization to enhance acidity and selectivity. Metal oxide catalysts commonly undergo sol-gel, co-precipitation, or combustion synthesis methods, with doping, calcination, and surface impregnation employed to tailor redox properties and catalytic activity. Both catalyst types benefit from post-synthesis modifications to optimize pore structure, active sites, and thermal stability for targeted reactions.
Environmental Impact and Regeneration
Zeolite catalysts exhibit superior environmental benefits due to their high selectivity and low toxicity, reducing harmful emissions during chemical reactions compared to metal oxide catalysts, which often generate more pollutants. The regeneration of zeolite catalysts is more efficient, involving mild thermal or chemical treatments that preserve their crystalline structure and catalytic activity, whereas metal oxide catalysts typically require harsher conditions, causing faster deactivation and increased waste. The sustainability of zeolite catalysts is further enhanced by their longer operational lifespan and lower environmental footprint throughout regeneration cycles.
Future Trends in Catalyst Development
Zeolite catalysts are increasingly favored for their high surface area and shape-selective properties, enabling enhanced efficiency in petrochemical and environmental applications. Metal oxide catalysts continue to evolve with improvements in thermal stability and redox activity, pivotal for oxidative reactions and emission control. Future trends emphasize hybrid catalyst systems combining zeolites and metal oxides to achieve superior catalytic performance, stability, and selectivity in sustainable chemical processes.
Acidic site density
Zeolite catalysts exhibit higher acidic site density compared to metal oxide catalysts, enabling enhanced catalytic activity in acid-driven reactions.
Redox activity
Zeolite catalysts exhibit selective acid-base properties with limited redox activity, whereas metal oxide catalysts demonstrate strong redox capabilities essential for oxidation-reduction reactions in industrial processes.
Shape selectivity
Zeolite catalysts exhibit superior shape selectivity due to their uniform microporous structures, whereas metal oxide catalysts generally lack this molecular-level discrimination.
Hydrothermal stability
Zeolite catalysts exhibit superior hydrothermal stability compared to metal oxide catalysts, maintaining structural integrity and catalytic performance under high temperature and moisture conditions.
Cation exchange capacity
Zeolite catalysts exhibit higher cation exchange capacity than metal oxide catalysts, enhancing their ion-exchange efficiency and catalytic performance in chemical reactions.
Isomorphous substitution
Zeolite catalysts exhibit enhanced acidity and catalytic performance due to isomorphous substitution of framework atoms, whereas metal oxide catalysts rely on surface defect sites without such framework substitutions.
Oxygen vacancy
Zeolite catalysts with controlled oxygen vacancies exhibit superior catalytic activity and selectivity compared to metal oxide catalysts due to enhanced oxygen mobility and electron transfer capabilities.
Framework topology
Zeolite catalysts feature a well-defined crystalline framework topology with uniform micropores that enhance shape selectivity, whereas metal oxide catalysts typically exhibit less ordered, amorphous structures with variable pore sizes impacting their catalytic performance.
Bifunctional catalysis
Zeolite catalysts offer superior bifunctional catalysis by combining acidic sites with shape-selective properties, whereas metal oxide catalysts primarily provide redox sites with limited acidity, impacting their performance in hydrocarbon conversion reactions.
Catalyst deactivation
Zeolite catalysts experience faster deactivation due to pore blockage and coke formation, whereas metal oxide catalysts primarily deactivate through surface sintering and phase transformations.
Zeolite catalyst vs Metal oxide catalyst Infographic
 njnir.com
njnir.com