Membrane distillation uses a hydrophobic membrane to separate components based on vapor pressure differences, making it ideal for desalination and wastewater treatment. Pervaporation relies on selective permeation followed by evaporation, effectively separating azeotropic mixtures and organic solvents. Both techniques offer energy-efficient separation but differ in mechanism and application scope within chemical engineering processes.
Table of Comparison
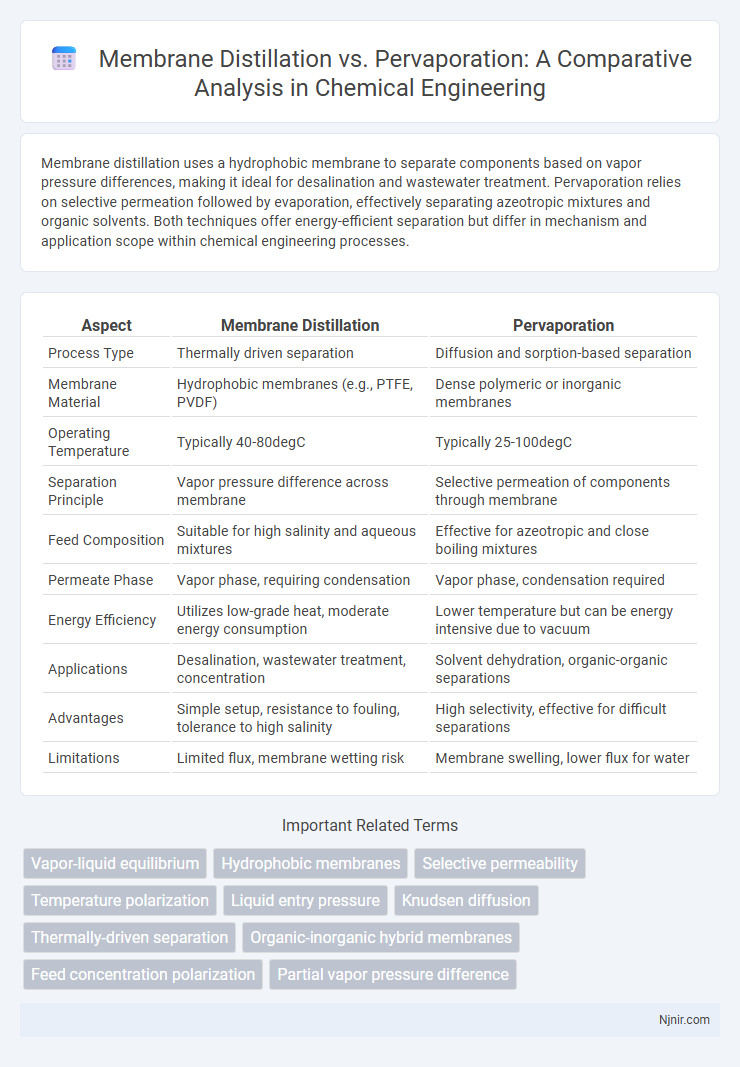
| Aspect | Membrane Distillation | Pervaporation |
|---|---|---|
| Process Type | Thermally driven separation | Diffusion and sorption-based separation |
| Membrane Material | Hydrophobic membranes (e.g., PTFE, PVDF) | Dense polymeric or inorganic membranes |
| Operating Temperature | Typically 40-80degC | Typically 25-100degC |
| Separation Principle | Vapor pressure difference across membrane | Selective permeation of components through membrane |
| Feed Composition | Suitable for high salinity and aqueous mixtures | Effective for azeotropic and close boiling mixtures |
| Permeate Phase | Vapor phase, requiring condensation | Vapor phase, condensation required |
| Energy Efficiency | Utilizes low-grade heat, moderate energy consumption | Lower temperature but can be energy intensive due to vacuum |
| Applications | Desalination, wastewater treatment, concentration | Solvent dehydration, organic-organic separations |
| Advantages | Simple setup, resistance to fouling, tolerance to high salinity | High selectivity, effective for difficult separations |
| Limitations | Limited flux, membrane wetting risk | Membrane swelling, lower flux for water |
Introduction to Membrane Distillation and Pervaporation
Membrane distillation utilizes a hydrophobic membrane to separate vapor from a liquid feed, driven by a temperature-induced vapor pressure difference. Pervaporation involves the selective permeation of components through a membrane followed by evaporation on the permeate side, targeting separation of azeotropic or close-boiling mixtures. Both techniques offer energy-efficient alternatives for liquid separation, with membrane distillation being ideal for desalination and wastewater treatment, while pervaporation excels in organic solvent dehydration and chemical recovery.
Fundamental Principles of Separation Processes
Membrane distillation relies on a hydrophobic microporous membrane that allows vapor phase transport driven by a vapor pressure gradient, enabling separation based on volatility differences. Pervaporation utilizes a dense, non-porous membrane where selective sorption and diffusion of components occur, enabling separation primarily based on solubility and diffusivity differences. Both processes leverage phase change and selective transport mechanisms, with membrane distillation focusing on vapor-based separation and pervaporation emphasizing liquid-phase diffusion selectivity.
Mechanisms of Membrane Distillation
Membrane distillation operates on a hydrophobic membrane that allows water vapor to pass while blocking liquid water, driven by a vapor pressure gradient across the membrane. This process relies on thermal energy, where heated feed water produces vapor that diffuses through the membrane pores and condenses on the cooler permeate side. Unlike pervaporation, which separates components based on selective sorption and diffusion through a dense membrane, membrane distillation leverages phase change and vapor transport for effective separation.
Pervaporation Working Principle
Pervaporation operates by selectively permeating volatile components from a liquid mixture through a dense, non-porous membrane, driven by a chemical potential gradient and maintained by vacuum or sweeping gas on the permeate side. This process separates mixtures based on differences in membrane affinity and vapor pressure between components, enabling efficient separation of azeotropes and close-boiling substances. In contrast, membrane distillation relies on a hydrophobic, microporous membrane and vapor pressure differences across the membrane, primarily targeting the separation of water from non-volatile solutes.
Material Selection for Membrane Technology
Membrane distillation and pervaporation differ significantly in material selection due to their distinct operating principles and separation needs. Membrane distillation requires hydrophobic membranes, often made from materials like PTFE, PVDF, or PP, which resist liquid water penetration while allowing vapor transport. In contrast, pervaporation membranes typically use dense, selective polymeric or ceramic materials tailored for specific solute permeability, emphasizing high selectivity and chemical affinity for target compounds.
Performance Comparison: Efficiency and Selectivity
Membrane distillation offers high thermal efficiency and is highly effective for separating volatile compounds by exploiting vapor pressure differences, achieving separation factors often exceeding 50. Pervaporation demonstrates superior selectivity for separating azeotropic mixtures and organic solvents due to its polymeric membrane affinity, with separation factors frequently surpassing 100. Efficiency in membrane distillation is influenced by temperature gradients, whereas pervaporation efficiency depends on membrane material and feed composition, making pervaporation more suitable for precise separations in petrochemical and pharmaceutical applications.
Applications in Industry: Case Studies
Membrane distillation is widely applied in desalination and wastewater treatment, offering energy-efficient separation of water from salts and contaminants in industries such as power generation and food processing. Pervaporation excels in organic solvent recovery and biofuel purification, demonstrated by case studies in petrochemical plants and pharmaceutical manufacturing where selective separation of azeotropic mixtures is critical. Industrial implementations highlight membrane distillation's advantage in handling high-salinity streams while pervaporation provides superior performance in separating close boiling point compounds and volatile organic compounds.
Energy Consumption and Sustainability
Membrane distillation typically operates at moderate temperatures and uses thermal energy, often derived from low-grade heat sources, resulting in moderate energy consumption suitable for sustainable applications. Pervaporation relies on vacuum and membrane selectivity to separate components, consuming electrical energy for vacuum pumps but often achieving higher separation efficiency and lower overall energy demand. Both technologies enhance sustainability by enabling water purification and solvent recovery with reduced chemical use and potential integration with renewable energy systems, but membrane distillation tends to leverage waste heat more effectively, while pervaporation offers energy savings through selective permeation.
Challenges and Limitations
Membrane distillation faces challenges such as membrane fouling, thermal polarization, and high energy consumption due to temperature gradients required for operation. Pervaporation limitations include membrane selectivity degradation over time, limited permeate flux, and sensitivity to feed composition variations affecting separation efficiency. Both technologies struggle with scalability and economic viability for large-scale industrial applications.
Future Trends in Membrane-Based Separation
Future trends in membrane-based separation highlight the increasing integration of membrane distillation with renewable energy sources to enhance desalination efficiency and sustainability. Advances in membrane materials, such as hydrophobic nanofibers and graphene-based composites, are driving improvements in selectivity and flux for both membrane distillation and pervaporation processes. Emerging hybrid systems combining membrane distillation and pervaporation offer promising solutions for complex separations in wastewater treatment and chemical recovery.
Vapor-liquid equilibrium
Membrane distillation leverages temperature-induced vapor-liquid equilibrium differences to separate components via hydrophobic membranes, whereas pervaporation relies on selective permeation and partial vaporization driven by chemical potential gradients across dense membranes.
Hydrophobic membranes
Hydrophobic membranes in membrane distillation enable effective separation by vapor transport through microporous structures, while in pervaporation, they selectively permeate volatile components via solution-diffusion mechanisms for separation of liquid mixtures.
Selective permeability
Membrane distillation exhibits high selective permeability for volatile compounds driven by vapor pressure differences, while pervaporation offers superior selectivity for separating liquid mixtures based on differential sorption and diffusion through dense membranes.
Temperature polarization
Membrane distillation experiences significant temperature polarization that reduces thermal efficiency, whereas pervaporation minimizes temperature polarization through selective vapor permeation at the membrane interface.
Liquid entry pressure
Membrane distillation requires a higher liquid entry pressure to prevent pore wetting, whereas pervaporation operates with dense membranes that do not rely on liquid entry pressure for selective vapor transport.
Knudsen diffusion
Membrane distillation utilizes Knudsen diffusion as the primary mass transfer mechanism in porous hydrophobic membranes, whereas pervaporation relies on solution-diffusion without Knudsen diffusion dominance.
Thermally-driven separation
Membrane distillation utilizes hydrophobic membranes and temperature gradients to separate volatile components via vapor pressure differences, while pervaporation employs dense, selective membranes enabling liquid-phase separation driven by chemical potential differences under thermal conditions.
Organic-inorganic hybrid membranes
Organic-inorganic hybrid membranes enhance separation efficiency and thermal stability in both membrane distillation and pervaporation processes by combining the hydrophobicity of organic polymers with the mechanical strength and selectivity of inorganic materials.
Feed concentration polarization
Membrane distillation experiences higher feed concentration polarization due to vapor flux limitations, whereas pervaporation minimizes this effect by selective permeation of components through a dense membrane.
Partial vapor pressure difference
Membrane distillation relies on a higher partial vapor pressure difference across a hydrophobic membrane to drive vapor transport, whereas pervaporation utilizes selective permeation combined with phase change, often operating with a smaller vapor pressure gradient but enhanced solute selectivity.
Membrane distillation vs Pervaporation Infographic
 njnir.com
njnir.com