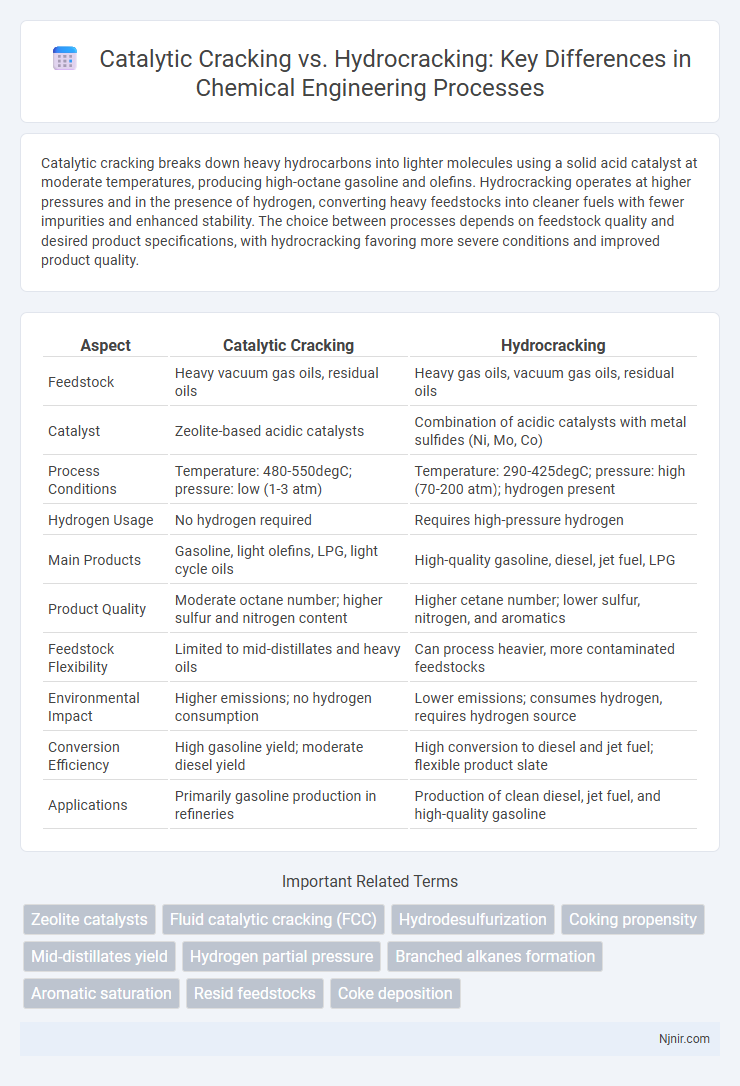
Catalytic cracking breaks down heavy hydrocarbons into lighter molecules using a solid acid catalyst at moderate temperatures, producing high-octane gasoline and olefins. Hydrocracking operates at higher pressures and in the presence of hydrogen, converting heavy feedstocks into cleaner fuels with fewer impurities and enhanced stability. The choice between processes depends on feedstock quality and desired product specifications, with hydrocracking favoring more severe conditions and improved product quality.
Table of Comparison
| Aspect | Catalytic Cracking | Hydrocracking |
|---|---|---|
| Feedstock | Heavy vacuum gas oils, residual oils | Heavy gas oils, vacuum gas oils, residual oils |
| Catalyst | Zeolite-based acidic catalysts | Combination of acidic catalysts with metal sulfides (Ni, Mo, Co) |
| Process Conditions | Temperature: 480-550degC; pressure: low (1-3 atm) | Temperature: 290-425degC; pressure: high (70-200 atm); hydrogen present |
| Hydrogen Usage | No hydrogen required | Requires high-pressure hydrogen |
| Main Products | Gasoline, light olefins, LPG, light cycle oils | High-quality gasoline, diesel, jet fuel, LPG |
| Product Quality | Moderate octane number; higher sulfur and nitrogen content | Higher cetane number; lower sulfur, nitrogen, and aromatics |
| Feedstock Flexibility | Limited to mid-distillates and heavy oils | Can process heavier, more contaminated feedstocks |
| Environmental Impact | Higher emissions; no hydrogen consumption | Lower emissions; consumes hydrogen, requires hydrogen source |
| Conversion Efficiency | High gasoline yield; moderate diesel yield | High conversion to diesel and jet fuel; flexible product slate |
| Applications | Primarily gasoline production in refineries | Production of clean diesel, jet fuel, and high-quality gasoline |
Introduction to Catalytic Cracking and Hydrocracking
Catalytic cracking uses a catalyst to break large hydrocarbon molecules into smaller, more valuable products like gasoline and diesel through thermal degradation and molecular rearrangement. Hydrocracking combines catalytic cracking with hydrogenation, operating under high pressure and hydrogen to produce cleaner fuels with higher yields of light hydrocarbons. Both processes are essential in refining crude oil but differ in feedstock flexibility, operating conditions, and product output quality.
Historical Development and Industrial Adoption
Catalytic cracking was developed in the 1930s as a breakthrough to efficiently convert heavy hydrocarbons into lighter, more valuable fuels like gasoline, revolutionizing the oil refining industry. Hydrocracking emerged in the 1950s, integrating hydrogenation with catalytic cracking to produce cleaner fuels and accommodate heavier feedstocks amid rising environmental regulations. Both technologies rapidly gained industrial adoption, with catalytic cracking becoming a cornerstone of mid-20th century refineries and hydrocracking expanding significantly in the late 20th century to meet demand for diesel and jet fuels.
Fundamental Principles of Catalytic Cracking
Catalytic cracking relies on zeolite catalysts to break long-chain hydrocarbons into shorter, more valuable fractions by utilizing acidic sites that promote carbocation formation and cleavage. The process operates at moderate temperatures (450-550degC) and low hydrogen pressure, favoring cracking reactions without significant hydrogenation. This contrasts with hydrocracking, which requires higher hydrogen pressure and metal catalysts to simultaneously crack and hydrogenate feedstocks, producing saturated hydrocarbons with less olefins and aromatics.
Core Mechanisms of Hydrocracking
Hydrocracking utilizes high-pressure hydrogen and a bifunctional catalyst combining acidic sites and metal sites to simultaneously crack heavy hydrocarbons and saturate olefins, enhancing fuel quality and yield. The core mechanism involves hydrogenation of aromatic rings and olefins followed by acid-catalyzed cracking, preventing coke formation and ensuring higher stability of the products. This contrasts with catalytic cracking, which relies mainly on acidic catalysts without hydrogen, leading to more olefinic and aromatic compounds but lower product hydrogen content.
Catalyst Selection and Process Conditions
Catalytic cracking utilizes zeolite-based catalysts operating at temperatures between 480-550degC and atmospheric pressure to maximize gasoline yield by cracking heavy hydrocarbons. Hydrocracking employs bifunctional catalysts containing noble metals like palladium or platinum on acidic supports, functioning at higher pressures (70-200 bar) and moderate temperatures (260-425degC) to promote hydrogenation and cracking simultaneously. Catalyst selection in catalytic cracking favors acidity and pore structure for cracking efficiency, while hydrocracking catalysts prioritize hydrogenation activity and stability under high-pressure hydrogen environments.
Feedstock Flexibility and Product Yields
Catalytic cracking excels in processing heavier feedstocks such as vacuum gas oils, producing high yields of gasoline and light olefins, while hydrocracking offers superior feedstock flexibility by processing a broader range of hydrocarbons including heavier and more sulfur-rich feeds. Hydrocracking produces higher yields of middle distillates like diesel and jet fuel with improved product quality due to hydrogen addition, whereas catalytic cracking favors lighter products but with lower hydrogen content and higher olefin concentrations. Refiners choose hydrocracking for cleaner fuels and higher-value middle distillates, while catalytic cracking remains essential for maximizing gasoline output from heavier crude fractions.
Energy Efficiency and Operating Conditions
Catalytic cracking operates at higher temperatures, typically 450-550degC, and lower pressures around 1-5 bar, offering moderate energy efficiency by breaking heavy hydrocarbons into lighter fractions through thermal cracking. Hydrocracking functions under more severe conditions, with temperatures between 260-425degC and high hydrogen pressures ranging from 80-200 bar, enhancing energy efficiency by producing cleaner fuels with higher yields and lower coke formation. The presence of hydrogen in hydrocracking reduces energy consumption in downstream processing and improves catalyst life, making it more energy-efficient despite its more demanding operating environment.
Environmental Impact and Byproduct Formation
Catalytic cracking generates higher amounts of environmentally harmful byproducts such as sulfur oxides and nitrogen oxides due to incomplete hydrocarbon breakdown, increasing air pollution. Hydrocracking produces fewer sulfur-containing compounds and has lower greenhouse gas emissions by operating under hydrogen-rich conditions that saturate hydrocarbons more completely. The reduced formation of coke and heavy residues in hydrocracking minimizes solid waste, making it a more sustainable refining process compared to catalytic cracking.
Technological Advancements and Innovations
Catalytic cracking technology has evolved with the introduction of fluid catalytic cracking (FCC) units that enhance gasoline yield and improve catalyst regeneration processes. Hydrocracking advancements leverage high-pressure hydrogen environments and improved bifunctional catalysts to increase the production of high-quality diesel and jet fuels while reducing sulfur and aromatic content. Innovations such as nanostructured catalysts and process integration with renewable feedstocks have further optimized both technologies for energy efficiency and environmental compliance.
Future Trends in Cracking Technologies
Future trends in cracking technologies emphasize enhancing catalyst efficiency and selectivity to improve yield quality and reduce environmental impact. Advances in catalytic cracking focus on integrating nanostructured catalysts and process intensification for lower capital costs and higher throughput. Hydrocracking innovations prioritize hydrogen management and catalyst regeneration to meet stricter fuel standards and support sustainable refining amid evolving energy demands.
Zeolite catalysts
Zeolite catalysts enhance catalytic cracking by promoting hydrocarbon cracking and isomerization, while hydrocracking uses zeolites combined with metals to facilitate hydrogenation and produce higher yields of saturated, high-quality fuels.
Fluid catalytic cracking (FCC)
Fluid catalytic cracking (FCC) efficiently breaks down heavy hydrocarbons into lighter fuels by using a catalyst and heat, while hydrocracking employs hydrogen and catalysts to simultaneously crack and saturate molecules, producing higher-quality fuels with less sulfur.
Hydrodesulfurization
Hydrocracking integrates hydrodesulfurization to effectively remove sulfur compounds from heavy petroleum fractions, producing cleaner fuels compared to catalytic cracking.
Coking propensity
Catalytic cracking exhibits a higher coking propensity due to its use of acidic zeolite catalysts that promote coke formation, whereas hydrocracking uses hydrogenation catalysts under high hydrogen pressure, significantly reducing coke buildup.
Mid-distillates yield
Hydrocracking produces a higher yield of mid-distillates such as diesel and jet fuel with improved sulfur removal compared to catalytic cracking, which primarily generates more gasoline and lighter products.
Hydrogen partial pressure
Hydrocracking operates under high hydrogen partial pressure to enhance saturation and minimize coke formation, whereas catalytic cracking functions at low or no hydrogen partial pressure, relying on acid catalysts for hydrocarbon breakdown without hydrogen addition.
Branched alkanes formation
Hydrocracking generates higher yields of branched alkanes due to hydrogenation and isomerization under high pressure and temperature, whereas catalytic cracking primarily produces linear alkanes and olefins through thermal decomposition without significant branching.
Aromatic saturation
Hydrocracking enhances aromatic saturation by adding hydrogen to break aromatic rings, whereas catalytic cracking primarily cracks hydrocarbons without significantly saturating aromatics.
Resid feedstocks
Catalytic cracking efficiently converts heavy resid feedstocks into lighter hydrocarbons using zeolite catalysts under moderate hydrogen absence, whereas hydrocracking processes resid feedstocks at high hydrogen pressures to produce cleaner, high-quality middle distillates with enhanced sulfur and nitrogen removal.
Coke deposition
Catalytic cracking produces higher coke deposition on catalysts due to severe thermal conditions, while hydrocracking minimizes coke formation by using hydrogen under milder conditions.
Catalytic cracking vs Hydrocracking Infographic
 njnir.com
njnir.com