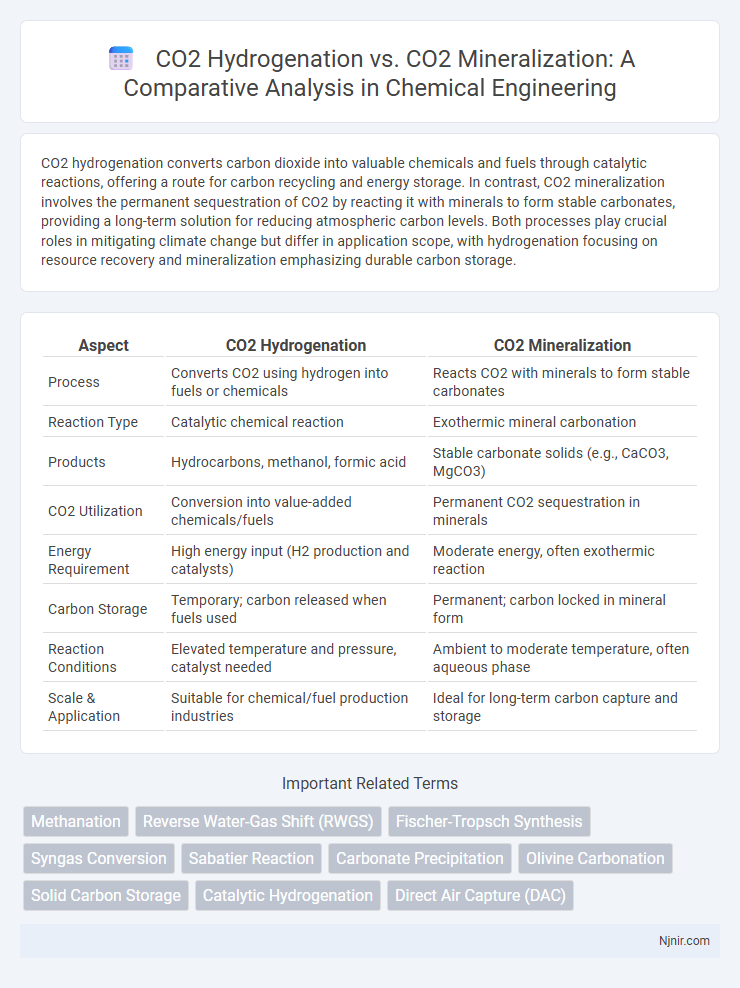
CO2 hydrogenation converts carbon dioxide into valuable chemicals and fuels through catalytic reactions, offering a route for carbon recycling and energy storage. In contrast, CO2 mineralization involves the permanent sequestration of CO2 by reacting it with minerals to form stable carbonates, providing a long-term solution for reducing atmospheric carbon levels. Both processes play crucial roles in mitigating climate change but differ in application scope, with hydrogenation focusing on resource recovery and mineralization emphasizing durable carbon storage.
Table of Comparison
| Aspect | CO2 Hydrogenation | CO2 Mineralization |
|---|---|---|
| Process | Converts CO2 using hydrogen into fuels or chemicals | Reacts CO2 with minerals to form stable carbonates |
| Reaction Type | Catalytic chemical reaction | Exothermic mineral carbonation |
| Products | Hydrocarbons, methanol, formic acid | Stable carbonate solids (e.g., CaCO3, MgCO3) |
| CO2 Utilization | Conversion into value-added chemicals/fuels | Permanent CO2 sequestration in minerals |
| Energy Requirement | High energy input (H2 production and catalysts) | Moderate energy, often exothermic reaction |
| Carbon Storage | Temporary; carbon released when fuels used | Permanent; carbon locked in mineral form |
| Reaction Conditions | Elevated temperature and pressure, catalyst needed | Ambient to moderate temperature, often aqueous phase |
| Scale & Application | Suitable for chemical/fuel production industries | Ideal for long-term carbon capture and storage |
Introduction to CO2 Utilization Technologies
CO2 hydrogenation and CO2 mineralization represent two pivotal CO2 utilization technologies aimed at mitigating greenhouse gas emissions. CO2 hydrogenation converts carbon dioxide into valuable chemicals and fuels using catalysts and hydrogen, effectively recycling CO2 into energy carriers like methanol. CO2 mineralization stabilizes CO2 by reacting it with minerals to form stable carbonates, offering permanent carbon sequestration while producing useful construction materials.
Overview of CO2 Hydrogenation
CO2 hydrogenation is a catalytic process that converts carbon dioxide into valuable chemicals such as methanol, methane, and other hydrocarbons using hydrogen gas. This technology leverages metal catalysts like copper, nickel, and ruthenium to enable efficient CO2 reduction under controlled temperature and pressure conditions. Compared to CO2 mineralization, which permanently sequesters CO2 in stable carbonate minerals, hydrogenation offers the dual benefit of carbon recycling and production of renewable fuels and chemicals.
Fundamentals of CO2 Mineralization
CO2 mineralization involves the chemical transformation of carbon dioxide into stable carbonate minerals through exothermic reactions with metal oxides, offering permanent carbon sequestration with minimal risk of re-release. This process is governed by factors such as mineral reactivity, temperature, pressure, and the availability of divalent cations like calcium or magnesium, which facilitate the formation of solid carbonates. Unlike CO2 hydrogenation, which converts CO2 into fuels or chemicals via catalytic reactions requiring hydrogen, CO2 mineralization emphasizes long-term storage by producing insoluble, stable compounds.
Reaction Mechanisms: Hydrogenation vs Mineralization
CO2 hydrogenation involves the catalytic conversion of carbon dioxide into valuable hydrocarbons or alcohols through the addition of hydrogen, typically utilizing metal catalysts such as Ni, Cu, or Ru to activate CO2 and H2 molecules. In contrast, CO2 mineralization entails the chemical reaction between CO2 and metal oxides or hydroxides, forming stable carbonate minerals like CaCO3 or MgCO3, which permanently sequester carbon in solid form. The hydrogenation mechanism relies on surface adsorption and stepwise hydrogen addition, while mineralization proceeds via acid-base reactions and crystal nucleation, resulting in fundamentally different pathways for CO2 utilization and storage.
Catalysts and Materials Used
CO2 hydrogenation primarily utilizes catalysts such as copper-based, nickel-based, and noble metal-supported catalysts (e.g., Pd, Pt) to facilitate conversion into hydrocarbons or methanol, while optimizing catalyst surface area and metal dispersion enhances activity and selectivity. In CO2 mineralization, materials like alkaline earth metal oxides (MgO, CaO) and silicate minerals (olivine, serpentine) serve as reactants to chemically bind CO2 into stable carbonate minerals, with additives such as activated carbon or zeolites improving CO2 adsorption and reaction kinetics. Advances in catalyst design for hydrogenation focus on bimetallic alloys and metal-support interactions, whereas mineralization research emphasizes high-surface-area substrates and engineered reaction environments for rapid carbonation.
Thermodynamic and Kinetic Considerations
CO2 hydrogenation involves converting CO2 into valuable hydrocarbons or alcohols using H2, driven by favorable thermodynamics at elevated temperatures and pressures, but often limited by slow kinetics requiring effective catalysts. In contrast, CO2 mineralization converts CO2 into stable carbonate minerals, offering highly exothermic thermodynamics and near-irreversible reactions, yet the process can be kinetically slow due to solid-phase diffusion limitations. Optimizing catalysts and reaction conditions is crucial for enhancing CO2 hydrogenation kinetics, while accelerating mineralization typically demands increased surface area or reactive mineral availability to overcome kinetic barriers.
Process Design and Engineering Challenges
CO2 hydrogenation involves catalytic conversion of CO2 into value-added chemicals such as methanol or hydrocarbons, requiring precise reactor design to optimize temperature, pressure, and catalyst efficiency while managing heat integration and reaction kinetics. CO2 mineralization, on the other hand, entails the exothermic reaction of CO2 with alkaline earth metals or industrial waste to form stable carbonates, posing challenges in feedstock handling, reaction rate enhancement, and scalability of continuous processing systems. Both processes demand advanced process integration, energy optimization, and materials selection to address mass transfer limitations, catalyst or mineral reactivity, and CO2 capture purity for viable industrial-scale implementation.
Energy Efficiency and Carbon Footprint Comparison
CO2 hydrogenation converts CO2 into valuable fuels or chemicals by using hydrogen, requiring significant energy input primarily from renewable sources to maintain high energy efficiency and lower carbon footprint compared to fossil-based processes. CO2 mineralization stabilizes CO2 by chemically transforming it into solid carbonates, offering a highly durable carbon sink with minimal energy demand and near-zero emissions, making it more energy-efficient and carbon-negative in certain applications. When comparing CO2 hydrogenation and mineralization, mineralization generally achieves lower carbon footprint due to its permanence and lower operational energy, while hydrogenation supports circular carbon economy objectives through fuel synthesis but demands higher energy input.
Industrial Applications and Case Studies
CO2 hydrogenation converts carbon dioxide into valuable chemicals like methanol and hydrocarbons using catalytic processes, with industrial applications in sustainable fuel production and chemical manufacturing exemplified by companies such as Carbon Recycling International. CO2 mineralization involves the reaction of CO2 with metal oxides to form stable carbonates, offering scalable solutions for permanent carbon storage demonstrated by projects like Carbfix in Iceland. Both methods contribute to carbon utilization strategies, where hydrogenation targets chemical feedstocks and fuels while mineralization emphasizes long-term sequestration in construction materials and geological formations.
Future Prospects and Research Directions
CO2 hydrogenation offers promising pathways for converting captured carbon dioxide into valuable chemicals and fuels, emphasizing catalyst development and process efficiency to enhance scalability and commercial viability. Research directions focus on optimizing reaction conditions, exploring novel catalytic materials like single-atom catalysts, and integrating renewable hydrogen sources to reduce carbon footprints. In contrast, CO2 mineralization aims for permanent carbon sequestration through stable carbonate formation, where advances in accelerated weathering techniques and alternative mineral feedstocks are pivotal for large-scale deployment and environmental sustainability.
Methanation
CO2 methanation efficiently converts carbon dioxide into methane using catalytic hydrogenation, offering a renewable energy storage method compared to CO2 mineralization, which permanently stores CO2 as solid carbonates but does not produce fuel.
Reverse Water-Gas Shift (RWGS)
The Reverse Water-Gas Shift (RWGS) reaction efficiently converts CO2 into CO as an intermediate for hydrogenation processes, offering a controlled pathway compared to the irreversible and stable product formation in CO2 mineralization.
Fischer-Tropsch Synthesis
CO2 hydrogenation via Fischer-Tropsch synthesis converts captured CO2 into valuable hydrocarbons using syngas derived from hydrogen and CO2, whereas CO2 mineralization permanently stabilizes CO2 by converting it into solid carbonates, emphasizing catalytic efficiency and product selectivity in FT processes for sustainable fuel production.
Syngas Conversion
CO2 hydrogenation converts CO2 into valuable syngas components (CO and H2) through catalytically driven reactions, whereas CO2 mineralization stabilizes CO2 into solid carbonates, making hydrogenation more effective for syngas conversion applications.
Sabatier Reaction
The Sabatier Reaction efficiently converts CO2 and hydrogen into methane and water through CO2 hydrogenation, offering a renewable energy storage method distinct from the permanent CO2 sequestration in mineralization.
Carbonate Precipitation
CO2 mineralization through carbonate precipitation offers a stable and permanent carbon storage method by converting CO2 into solid carbonate minerals, contrasting with CO2 hydrogenation which produces fuels or chemicals but requires continuous energy input.
Olivine Carbonation
Olivine carbonation, a CO2 mineralization process, offers a stable and long-term carbon storage solution by chemically binding CO2 into solid carbonate minerals, contrasting with CO2 hydrogenation which converts CO2 into valuable chemicals but requires continuous energy input.
Solid Carbon Storage
CO2 hydrogenation converts carbon dioxide into valuable hydrocarbons, whereas CO2 mineralization stabilizes carbon by transforming it into solid carbonates, making mineralization more effective for long-term solid carbon storage.
Catalytic Hydrogenation
Catalytic hydrogenation of CO2 efficiently converts carbon dioxide into valuable hydrocarbons and alcohols using metal catalysts, offering a renewable route for carbon utilization compared to the stable, long-term carbon sequestration achieved through CO2 mineralization.
Direct Air Capture (DAC)
Direct Air Capture (DAC) leverages CO2 hydrogenation to convert captured atmospheric CO2 into synthetic fuels, whereas CO2 mineralization permanently stores CO2 as stable carbonate minerals, providing distinct pathways for carbon management.
CO2 hydrogenation vs CO2 mineralization Infographic
 njnir.com
njnir.com