Heterogeneous catalysis involves catalysts in a different phase than the reactants, typically solid catalysts interacting with liquid or gas reactants, enhancing separation and reuse efficiency. Homogeneous catalysis features catalysts and reactants in the same phase, often liquid, offering superior selectivity and reaction control. The choice between heterogeneous and homogeneous catalysis depends on factors such as reaction conditions, catalyst recovery, and desired product specificity.
Table of Comparison
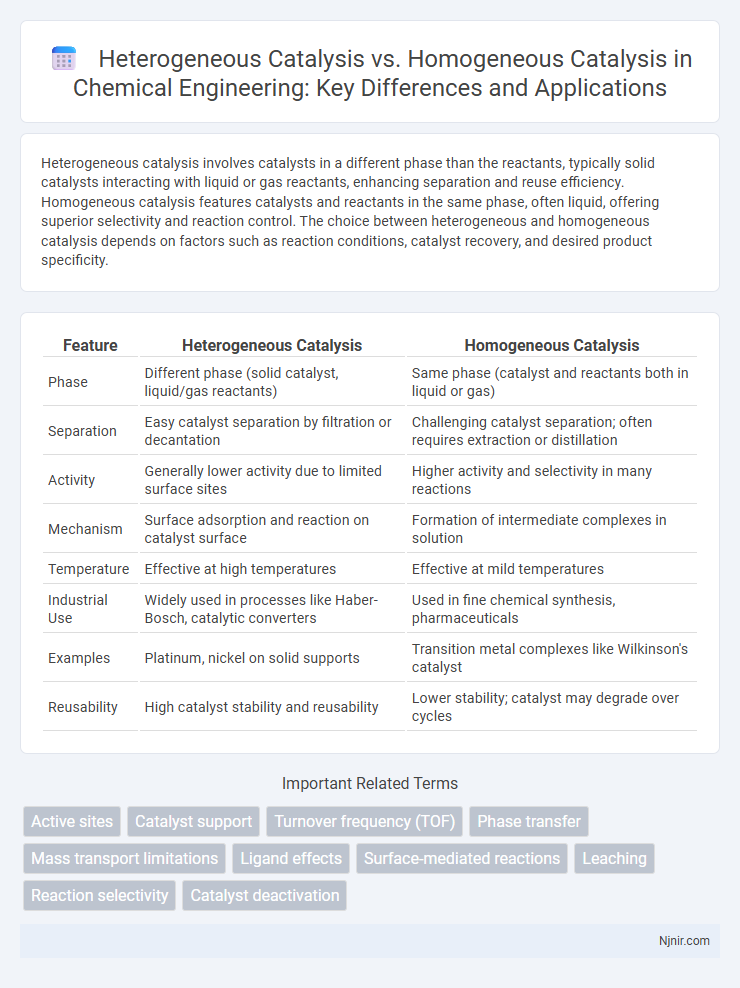
| Feature | Heterogeneous Catalysis | Homogeneous Catalysis |
|---|---|---|
| Phase | Different phase (solid catalyst, liquid/gas reactants) | Same phase (catalyst and reactants both in liquid or gas) |
| Separation | Easy catalyst separation by filtration or decantation | Challenging catalyst separation; often requires extraction or distillation |
| Activity | Generally lower activity due to limited surface sites | Higher activity and selectivity in many reactions |
| Mechanism | Surface adsorption and reaction on catalyst surface | Formation of intermediate complexes in solution |
| Temperature | Effective at high temperatures | Effective at mild temperatures |
| Industrial Use | Widely used in processes like Haber-Bosch, catalytic converters | Used in fine chemical synthesis, pharmaceuticals |
| Examples | Platinum, nickel on solid supports | Transition metal complexes like Wilkinson's catalyst |
| Reusability | High catalyst stability and reusability | Lower stability; catalyst may degrade over cycles |
Introduction to Catalysis in Chemical Engineering
Heterogeneous catalysis involves catalysts in a different phase than the reactants, typically solid catalysts with liquid or gas reactants, enabling easy separation and catalyst recycling. Homogeneous catalysis uses catalysts in the same phase as the reactants, often liquid phase, offering high selectivity and uniform active sites for precise molecular interactions. In chemical engineering, understanding phase behavior, mass transfer limitations, and catalyst surface properties is critical for optimizing catalytic reaction rates and process efficiencies.
Fundamental Principles of Heterogeneous Catalysis
Heterogeneous catalysis involves reactions where the catalyst is in a different phase, typically solid, interacting with gaseous or liquid reactants on its surface. The fundamental principles include adsorption of reactants onto active sites, surface diffusion, reaction at these sites, and desorption of products, governed by factors like surface area, catalyst morphology, and electronic structure. These surface phenomena are crucial for catalytic efficiency, distinguishing heterogeneous catalysis from homogeneous catalysis where catalysts and reactants share the same phase.
Core Concepts in Homogeneous Catalysis
Homogeneous catalysis involves catalysts and reactants in the same phase, usually liquid, enabling precise molecular interactions that enhance selectivity and reaction rates. Core concepts include catalyst coordination chemistry, where transition metal complexes facilitate bond activation and formation through well-defined ligand environments. This contrasts with heterogeneous catalysis, where catalysts are in a different phase, typically solid, and rely on surface interactions rather than molecular coordination.
Catalyst Structure and Mechanism Differences
Heterogeneous catalysis involves solid catalysts with active sites on surfaces interacting with reactants in different phases, enabling adsorption, surface reactions, and desorption processes. Homogeneous catalysis features catalysts in the same phase as reactants, typically dissolved molecules or ions, facilitating uniform distribution and molecular-level interactions through coordination complexes. Structural differences impact mechanisms where heterogeneous catalysis relies on surface phenomena and site accessibility, while homogeneous catalysis depends on well-defined coordination environments for selective activation.
Industrial Applications: Heterogeneous vs. Homogeneous Catalysis
Heterogeneous catalysis is widely favored in industrial applications due to its ease of separation, catalyst reuse, and operation under harsh conditions, making it ideal for processes like ammonia synthesis and petroleum refining. Homogeneous catalysis, offering superior selectivity and activity in organic synthesis, is essential in fine chemical production and pharmaceutical manufacturing despite challenges in catalyst recovery. Industrial adoption balances these factors by selecting heterogeneous catalysts for large-scale, robust processes and homogeneous catalysts for precision-driven reactions requiring high stereochemical control.
Advantages and Limitations of Heterogeneous Catalysts
Heterogeneous catalysts offer advantages such as easy separation from reaction mixtures, enhanced stability under harsh reaction conditions, and reusability, making them ideal for industrial processes like petrochemical refining and pollution control. However, their limitations include lower selectivity compared to homogeneous catalysts, limited active site accessibility due to surface area constraints, and mass transfer limitations that can reduce overall reaction rates. Despite these challenges, advancements in catalyst design and nanotechnology are improving the efficiency and applicability of heterogeneous catalysts in various chemical transformations.
Benefits and Challenges of Homogeneous Catalysts
Homogeneous catalysis offers distinct benefits such as high selectivity and ease of tuning catalyst properties at the molecular level, enabling precise control over reaction pathways and product distribution. However, challenges include catalyst recovery and separation from reaction mixtures, as well as limited thermal stability under harsh conditions compared to heterogeneous catalysts. Developing recyclable homogeneous catalysts with enhanced robustness remains a key focus in advancing catalytic processes.
Catalyst Separation and Recycling Methods
Heterogeneous catalysis offers straightforward catalyst separation and recycling through filtration, centrifugation, or magnetic separation due to the catalyst's solid phase contrasting with liquid reactants. Homogeneous catalysis requires advanced techniques such as solvent extraction, phase separation, or membrane filtration because the catalyst and reactants share the same phase, complicating isolation. Efficient catalyst recovery in both systems is crucial for cost-effectiveness and sustainability in industrial chemical processes.
Recent Innovations in Catalysis Technologies
Recent innovations in heterogeneous catalysis emphasize the development of nanostructured catalysts with enhanced surface area and active sites, improving reaction efficiency and selectivity for industrial processes. Advancements in homogeneous catalysis focus on designing robust organometallic complexes and ligand frameworks that enable precise control over catalytic cycles and facilitate greener, sustainable chemical transformations. Integration of machine learning and high-throughput screening accelerates the discovery and optimization of both catalyst types, driving breakthroughs in energy conversion and pharmaceutical synthesis.
Future Trends in Catalytic Process Development
Future trends in catalytic process development emphasize the integration of heterogeneous catalysis with advanced nanomaterials to enhance catalyst stability, selectivity, and recyclability. Homogeneous catalysis advances focus on designing ligands and metal complexes for improved reaction specificity and catalytic efficiency under milder conditions. Emerging hybrid catalytic systems combining the strengths of both heterogeneous and homogeneous catalysis aim to maximize performance in sustainable chemical manufacturing and renewable energy applications.
Active sites
Heterogeneous catalysis features active sites on solid surfaces enabling easy separation, while homogeneous catalysis occurs at molecularly dispersed active sites within the same phase as reactants, enhancing selectivity and uniformity.
Catalyst support
Heterogeneous catalysis utilizes solid catalyst supports to enhance surface area and facilitate reactant adsorption, while homogeneous catalysis operates in a single-phase solution without the need for supports, enabling uniform catalyst distribution and easier molecular interaction.
Turnover frequency (TOF)
Heterogeneous catalysis typically exhibits lower turnover frequency (TOF) due to limited active site accessibility compared to homogeneous catalysis, where molecular dispersion enables higher TOF values.
Phase transfer
Phase transfer in heterogeneous catalysis involves reactants moving between distinct solid and fluid phases, enhancing reaction rates while homogeneous catalysis occurs within a single phase, often leading to faster molecular interactions but requiring effective mixing to maintain phase uniformity.
Mass transport limitations
Heterogeneous catalysis often experiences mass transport limitations due to reactants diffusing through solid catalysts, whereas homogeneous catalysis typically exhibits fewer such limitations as reactants and catalysts are in the same phase, enhancing molecular interactions.
Ligand effects
Ligand effects in homogeneous catalysis modulate electronic and steric properties of metal centers, enhancing selectivity and activity, whereas heterogeneous catalysis typically lacks discrete ligands, relying on surface interactions and adsorption phenomena for catalytic performance.
Surface-mediated reactions
Surface-mediated reactions in heterogeneous catalysis occur on solid catalyst surfaces enabling easy separation and reuse, whereas homogeneous catalysis involves soluble catalysts that operate uniformly in the reaction mixture but pose challenges in catalyst recovery.
Leaching
Leaching in heterogeneous catalysis leads to metal loss and decreased catalyst stability, while homogeneous catalysis inherently involves soluble catalysts that do not suffer from leaching-induced deactivation.
Reaction selectivity
Heterogeneous catalysis offers higher reaction selectivity due to distinct active sites on solid catalysts, while homogeneous catalysis provides greater molecular-level control for fine-tuning selectivity in solution-phase reactions.
Catalyst deactivation
Catalyst deactivation in heterogeneous catalysis typically results from surface poisoning, sintering, or coking, whereas in homogeneous catalysis, it often arises from catalyst degradation, ligand dissociation, or metal complex aggregation.
heterogeneous catalysis vs homogeneous catalysis Infographic
 njnir.com
njnir.com