Supercritical fluid extraction (SFE) offers enhanced selectivity and environmentally friendly operation compared to traditional solvent extraction by utilizing supercritical CO2 as a green solvent. SFE operates at moderate temperatures and pressures, preserving thermally sensitive compounds while eliminating toxic solvent residues. This technique provides higher efficiency, faster processing times, and easier solvent recovery, making it a superior alternative for extracting bioactive compounds and essential oils.
Table of Comparison
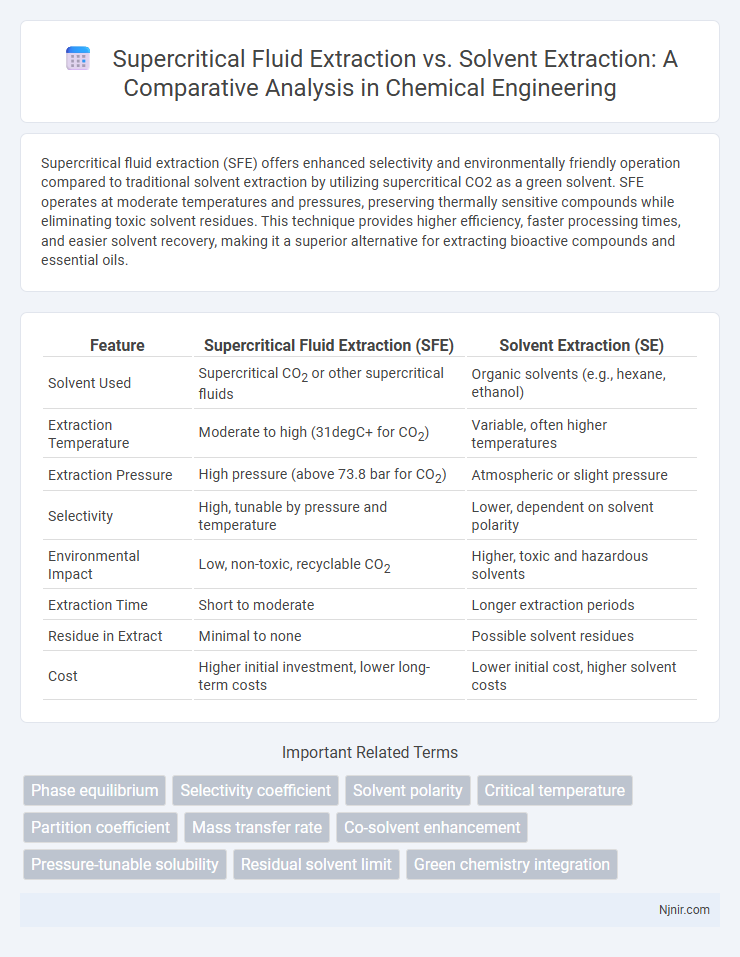
| Feature | Supercritical Fluid Extraction (SFE) | Solvent Extraction (SE) |
|---|---|---|
| Solvent Used | Supercritical CO2 or other supercritical fluids | Organic solvents (e.g., hexane, ethanol) |
| Extraction Temperature | Moderate to high (31degC+ for CO2) | Variable, often higher temperatures |
| Extraction Pressure | High pressure (above 73.8 bar for CO2) | Atmospheric or slight pressure |
| Selectivity | High, tunable by pressure and temperature | Lower, dependent on solvent polarity |
| Environmental Impact | Low, non-toxic, recyclable CO2 | Higher, toxic and hazardous solvents |
| Extraction Time | Short to moderate | Longer extraction periods |
| Residue in Extract | Minimal to none | Possible solvent residues |
| Cost | Higher initial investment, lower long-term costs | Lower initial cost, higher solvent costs |
Introduction to Extraction Techniques in Chemical Engineering
Supercritical fluid extraction (SFE) utilizes supercritical CO2 as a solvent to efficiently separate compounds at precise temperature and pressure conditions, offering eco-friendly and solvent-free alternatives compared to traditional solvent extraction. Solvent extraction relies on organic solvents to dissolve and separate components based on their solubility differences, often requiring longer processing times and generating hazardous waste. Chemical engineers optimize these extraction techniques by balancing factors such as selectivity, yield, environmental impact, and operational costs to enhance separation efficiency in various industrial applications.
Fundamentals of Supercritical Fluid Extraction (SFE)
Supercritical Fluid Extraction (SFE) utilizes fluids at temperatures and pressures above their critical points, where they exhibit unique solvent properties combining gas-like diffusivity and liquid-like density, enabling efficient extraction of target compounds. Carbon dioxide is the most commonly used supercritical fluid due to its non-toxicity, moderate critical temperature (31.1degC), and pressure (73.8 bar), making SFE suitable for heat-sensitive materials. Compared to traditional solvent extraction, SFE offers enhanced selectivity, faster mass transfer, and eliminates the need for toxic organic solvents, resulting in purer extracts and environmentally safer processes.
Principles of Solvent Extraction Methods
Solvent extraction methods rely on the differential solubility of compounds in two immiscible liquids, typically involving an organic solvent and an aqueous phase to separate target substances based on their chemical affinities. Supercritical fluid extraction uses supercritical fluids like CO2 above their critical temperature and pressure, enabling tunable solvating power and enhanced mass transfer rates for efficient extraction. Unlike traditional solvent extraction, supercritical fluid extraction offers selective solubility without residual solvents, making it ideal for heat-sensitive or high-purity applications.
Comparative Mechanisms: SFE vs Solvent Extraction
Supercritical fluid extraction (SFE) utilizes supercritical CO2 as a solvent, exploiting its tunable density and diffusivity to selectively dissolve target compounds under specific temperature and pressure conditions. In contrast, solvent extraction relies on the solubility differences of compounds in liquid solvents, often requiring organic chemicals that may leave residual solvents and generate hazardous waste. SFE offers enhanced mass transfer rates and environmental benefits due to its non-toxic, non-flammable supercritical fluids, whereas traditional solvent extraction processes can face limitations in selectivity and longer extraction times.
Solvent Selection and Environmental Impacts
Supercritical fluid extraction primarily uses supercritical CO2, a non-toxic, non-flammable solvent with low environmental impact and excellent solvent power for non-polar compounds, whereas solvent extraction depends on organic solvents like hexane or methanol, which may pose toxicity and disposal challenges. The selectivity of supercritical fluid extraction can be fine-tuned by adjusting pressure and temperature, reducing solvent residues and minimizing hazardous waste compared to conventional solvent extraction. Environmentally, supercritical fluid extraction offers a greener alternative by eliminating volatile organic compounds (VOCs) emissions and lowering overall solvent consumption, aligning with sustainable processing goals.
Extraction Efficiency and Selectivity
Supercritical fluid extraction (SFE) exhibits higher extraction efficiency and selectivity compared to traditional solvent extraction due to its tunable solvating power by adjusting pressure and temperature. SFE, using supercritical CO2, enables selective targeting of non-polar compounds with minimal co-extraction of impurities, resulting in purer extracts. Solvent extraction often suffers from lower selectivity and requires extensive purification to remove residual solvents and unwanted components.
Industrial Applications of SFE and Solvent Extraction
Supercritical fluid extraction (SFE) offers superior selectivity and environmental benefits in industrial applications such as food processing, pharmaceuticals, and essential oil extraction due to its use of non-toxic CO2 as a solvent. Solvent extraction remains prevalent in industries like metal recovery, petrochemicals, and natural product isolation because of its cost-effectiveness and scalability despite the use of potentially hazardous organic solvents. Innovations in SFE technology increasingly optimize yield and purity, driving its adoption where solvent residues and processing times are critical concerns in large-scale manufacturing.
Safety and Regulatory Considerations
Supercritical fluid extraction (SFE) offers enhanced safety by using non-toxic CO2 as the primary solvent, eliminating flammable or hazardous chemical risks commonly associated with traditional solvent extraction using organic solvents like hexane or ethanol. Regulatory agencies favor SFE due to its environmentally friendly profile and minimal solvent residues, simplifying compliance with stringent food and pharmaceutical safety standards. Solvent extraction requires rigorous handling protocols and disposal measures to manage toxic waste and volatile organic compounds, increasing regulatory burden and workplace hazards.
Economic Analysis and Operational Costs
Supercritical fluid extraction (SFE) typically involves higher initial capital investment due to specialized equipment and high-pressure operation, but it can lead to lower operational costs over time by reducing solvent consumption and waste disposal fees. Solvent extraction generally has lower upfront costs but incurs ongoing expenses related to solvent purchase, recovery, and environmental compliance, which can increase total operational costs. Economic analysis often favors SFE in long-term applications where solvent savings and improved product purity offset the higher initial expenditure.
Future Trends and Innovations in Extraction Technologies
Supercritical fluid extraction (SFE) is rapidly advancing with innovations such as tunable solvents and greener CO2-based systems, enhancing selectivity and reducing environmental impact compared to traditional solvent extraction methods. Future trends emphasize integrating artificial intelligence for process optimization and scaling up continuous SFE systems to achieve higher efficiency and sustainability in natural product extraction. Emerging hybrid techniques combining SFE with membrane filtration promise improved purity and extraction yields, positioning SFE as a dominant green extraction technology over conventional solvent-based approaches.
Phase equilibrium
Phase equilibrium in supercritical fluid extraction allows precise control of solute solubility and selectivity compared to the fixed phase boundaries in traditional solvent extraction, enhancing extraction efficiency and purity.
Selectivity coefficient
Supercritical fluid extraction exhibits a higher selectivity coefficient than solvent extraction, enabling more precise target compound isolation due to tunable solvent properties and reduced co-extraction of impurities.
Solvent polarity
Supercritical fluid extraction utilizes supercritical CO2 with tunable polarity, offering selective solubility advantages over fixed-polarity solvent extraction methods.
Critical temperature
Supercritical fluid extraction operates above the solvent's critical temperature, enhancing solubility and diffusion rates compared to traditional solvent extraction conducted below critical temperature.
Partition coefficient
Supercritical fluid extraction offers a tunable partition coefficient for selective solute separation, outperforming traditional solvent extraction by enhancing both efficiency and purity.
Mass transfer rate
Supercritical fluid extraction achieves a higher mass transfer rate than solvent extraction due to its lower viscosity and higher diffusivity, enhancing solute penetration and recovery efficiency.
Co-solvent enhancement
Supercritical fluid extraction with co-solvent enhancement significantly improves the solubility and selectivity of target compounds compared to traditional solvent extraction methods.
Pressure-tunable solubility
Supercritical fluid extraction offers pressure-tunable solubility that enhances selectivity and efficiency compared to fixed-solubility solvent extraction methods.
Residual solvent limit
Supercritical fluid extraction significantly reduces residual solvent limits compared to traditional solvent extraction, enhancing product purity and safety.
Green chemistry integration
Supercritical fluid extraction utilizes non-toxic CO2 as a green solvent, reducing hazardous waste and energy consumption compared to traditional solvent extraction methods in green chemistry applications.
supercritical fluid extraction vs solvent extraction Infographic
 njnir.com
njnir.com