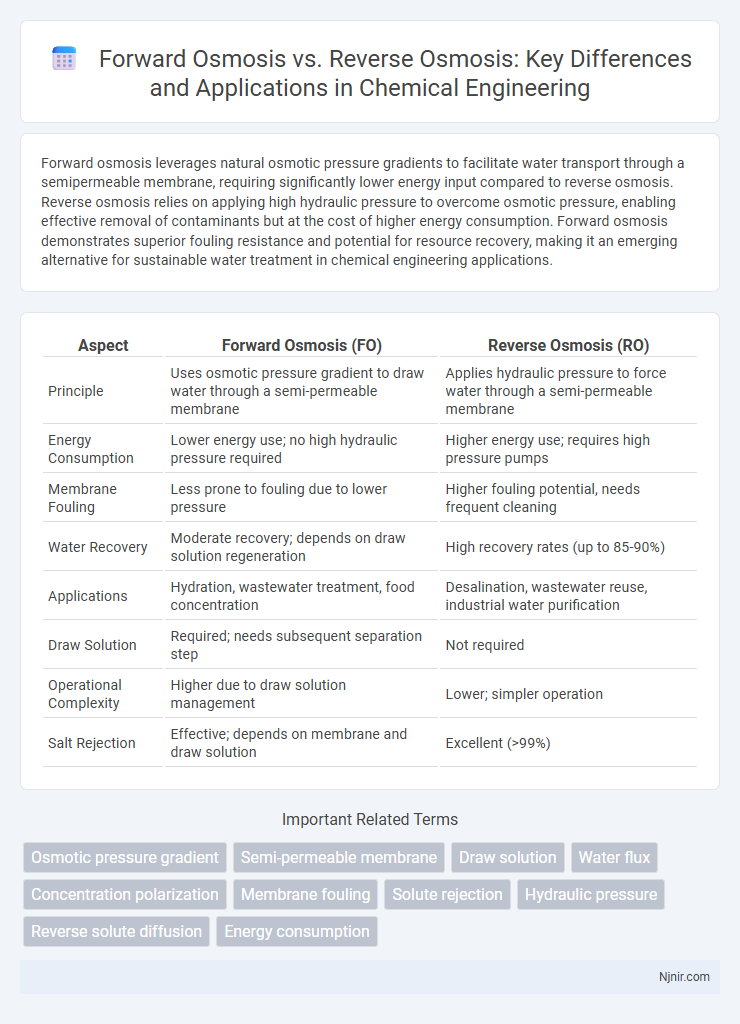
Forward osmosis leverages natural osmotic pressure gradients to facilitate water transport through a semipermeable membrane, requiring significantly lower energy input compared to reverse osmosis. Reverse osmosis relies on applying high hydraulic pressure to overcome osmotic pressure, enabling effective removal of contaminants but at the cost of higher energy consumption. Forward osmosis demonstrates superior fouling resistance and potential for resource recovery, making it an emerging alternative for sustainable water treatment in chemical engineering applications.
Table of Comparison
| Aspect | Forward Osmosis (FO) | Reverse Osmosis (RO) |
|---|---|---|
| Principle | Uses osmotic pressure gradient to draw water through a semi-permeable membrane | Applies hydraulic pressure to force water through a semi-permeable membrane |
| Energy Consumption | Lower energy use; no high hydraulic pressure required | Higher energy use; requires high pressure pumps |
| Membrane Fouling | Less prone to fouling due to lower pressure | Higher fouling potential, needs frequent cleaning |
| Water Recovery | Moderate recovery; depends on draw solution regeneration | High recovery rates (up to 85-90%) |
| Applications | Hydration, wastewater treatment, food concentration | Desalination, wastewater reuse, industrial water purification |
| Draw Solution | Required; needs subsequent separation step | Not required |
| Operational Complexity | Higher due to draw solution management | Lower; simpler operation |
| Salt Rejection | Effective; depends on membrane and draw solution | Excellent (>99%) |
Overview of Membrane Filtration in Chemical Engineering
Forward osmosis and reverse osmosis are membrane filtration processes widely applied in chemical engineering for separating solutes from solvents using selective membranes. Forward osmosis leverages osmotic pressure gradients to naturally draw water through a semi-permeable membrane, optimizing energy efficiency and reducing fouling risks. Reverse osmosis applies externally imposed pressure exceeding osmotic pressure to force solvent molecules through dense membranes, achieving high contaminant rejection essential for water purification and industrial separation processes.
Principles of Forward Osmosis (FO)
Forward osmosis (FO) operates on the principle of natural osmotic pressure difference, where water molecules pass through a semi-permeable membrane from a low osmotic pressure feed solution to a higher osmotic pressure draw solution without external hydraulic pressure. The membrane selectively allows water to flow while rejecting contaminants such as salts, organic compounds, and pathogens. FO's energy efficiency and reduced fouling potential distinguish it from reverse osmosis (RO), which relies on high-pressure pumps to force water through membranes.
Principles of Reverse Osmosis (RO)
Reverse osmosis (RO) operates on the principle of applying hydraulic pressure to overcome osmotic pressure, forcing water molecules through a semipermeable membrane while rejecting contaminants such as salts, bacteria, and organic compounds. The RO membrane acts as a selective barrier, allowing only pure water to pass while concentrating impurities on the feed side. This pressure-driven separation process is widely used in desalination, wastewater treatment, and purification of drinking water due to its high efficiency and effectiveness in removing a broad spectrum of dissolved solids.
Membrane Materials and Design for FO and RO
Forward osmosis (FO) membranes typically use asymmetric structures composed of a dense active layer and a porous support layer, often fabricated from cellulose triacetate (CTA) or thin-film composite (TFC) materials designed to minimize internal concentration polarization. Reverse osmosis (RO) membranes predominantly utilize thin-film composite polyamide materials featuring a dense selective layer atop a porous polysulfone substrate, optimized for high salt rejection and hydraulic pressure tolerance. The membrane design in FO focuses on maximizing water flux under osmotic gradients without high pressure, whereas RO membranes are engineered for mechanical strength and resistance to fouling under elevated operating pressures.
Comparative Energy Requirements: FO vs. RO
Forward osmosis (FO) requires significantly less energy than reverse osmosis (RO) due to its reliance on natural osmotic pressure rather than high hydraulic pressure. RO systems typically consume 3 to 10 times more energy, as they need powerful pumps to overcome osmotic gradients and force water through semi-permeable membranes. This energy efficiency makes FO a promising technology for low-energy water purification and desalination applications.
Solute Rejection and Water Recovery Efficiency
Forward osmosis (FO) typically achieves higher solute rejection rates due to its reliance on natural osmotic pressure gradients, effectively minimizing solute passage through the membrane. Reverse osmosis (RO), driven by hydraulic pressure, also provides high solute rejection but may face limitations with certain low-molecular-weight solutes. Water recovery efficiency in FO tends to be superior since it operates at lower pressures, reducing fouling and energy consumption, while RO systems often require higher pressure inputs, influencing their overall recovery efficiency and operational costs.
Application Areas: Industrial Use Cases of FO and RO
Forward osmosis (FO) is utilized in industrial applications such as wastewater treatment, food and beverage processing, and desalination where low energy consumption and fouling resistance are critical. Reverse osmosis (RO) is widely applied in water purification industries, including pharmaceutical manufacturing, power plants, and municipal water treatment due to its high contaminant rejection efficiency. Both technologies serve complementary roles in industrial settings, with FO preferred for energy-efficient pre-concentration and RO for producing high-purity water.
Operational Challenges and Fouling Mechanisms
Forward osmosis (FO) experiences lower hydraulic pressure than reverse osmosis (RO), yet faces challenges in draw solution recovery and concentration polarization, which intensifies fouling risks. RO systems operate under high pressure, making them prone to membrane compaction and scaling, with fouling primarily caused by particulate matter, biofilms, and inorganic salts. Both FO and RO require advanced cleaning protocols to mitigate fouling, but FO's fouling tends to be reversible, whereas RO fouling often demands chemical cleaning due to irreversible membrane damage.
Sustainability and Environmental Impact Assessment
Forward osmosis demonstrates a lower energy footprint compared to reverse osmosis, utilizing osmotic pressure instead of high hydraulic pressure, which reduces greenhouse gas emissions. The membrane fouling rate in forward osmosis is typically lower, leading to extended membrane lifespan and decreased chemical cleaning requirements, enhancing environmental sustainability. Reverse osmosis, while effective in contaminant removal, often generates higher brine concentrations that pose disposal challenges, making forward osmosis a more environmentally friendly option in water treatment and desalination processes.
Future Trends and Innovations in Osmosis Technologies
Future trends in osmosis technologies emphasize enhancing energy efficiency and sustainability, with forward osmosis (FO) gaining traction due to its lower energy consumption compared to reverse osmosis (RO). Innovations include the development of advanced selective membranes and hybrid systems combining FO and RO to optimize water recovery and reduce fouling. Research also focuses on integrating forward osmosis with renewable energy sources and wastewater treatment applications to expand its environmental benefits and commercial viability.
Osmotic pressure gradient
Forward osmosis utilizes a natural osmotic pressure gradient to draw water through a semipermeable membrane, whereas reverse osmosis applies an external pressure exceeding the natural osmotic gradient to force water separation.
Semi-permeable membrane
Forward osmosis utilizes a semi-permeable membrane to naturally draw water across by osmotic pressure without external pressure, whereas reverse osmosis relies on high external pressure to force water through a semi-permeable membrane, separating contaminants.
Draw solution
Forward osmosis utilizes a draw solution with high osmotic pressure to naturally pull water through a semi-permeable membrane, whereas reverse osmosis relies on high hydraulic pressure to force water against a membrane, making the draw solution critical for enhancing FO efficiency and selectivity.
Water flux
Forward osmosis achieves higher water flux at lower hydraulic pressure compared to reverse osmosis, making it more energy-efficient for desalination and wastewater treatment.
Concentration polarization
Forward osmosis experiences lower concentration polarization due to its natural osmotic pressure gradient, whereas reverse osmosis often suffers from severe concentration polarization caused by high hydraulic pressure.
Membrane fouling
Forward osmosis experiences less membrane fouling compared to reverse osmosis due to its lower hydraulic pressure and natural osmotic gradient operation.
Solute rejection
Forward osmosis demonstrates higher solute rejection rates than reverse osmosis by utilizing osmotic pressure gradients that minimize membrane fouling and allow selective water transport.
Hydraulic pressure
Forward osmosis operates with low hydraulic pressure by utilizing osmotic gradients, whereas reverse osmosis requires high hydraulic pressure to force water through semipermeable membranes.
Reverse solute diffusion
Reverse solute diffusion in forward osmosis leads to solute contamination of the feed solution, whereas reverse osmosis minimizes solute passage through its dense membrane, ensuring higher water purity.
Energy consumption
Forward osmosis consumes significantly less energy than reverse osmosis by relying on natural osmotic pressure instead of high-pressure pumps for water filtration.
forward osmosis vs reverse osmosis Infographic
 njnir.com
njnir.com