Pervaporation separates mixtures by selective permeation through a membrane and subsequent evaporation, making it highly effective for removing organic solvents from aqueous solutions. Reverse osmosis relies on applying high pressure to force water through a semi-permeable membrane, effectively desalinating or purifying water but often requiring higher energy input. Pervaporation offers advantages in energy efficiency and selectivity for azeotropic or close-boiling mixtures compared to reverse osmosis, which excels at large-scale water purification.
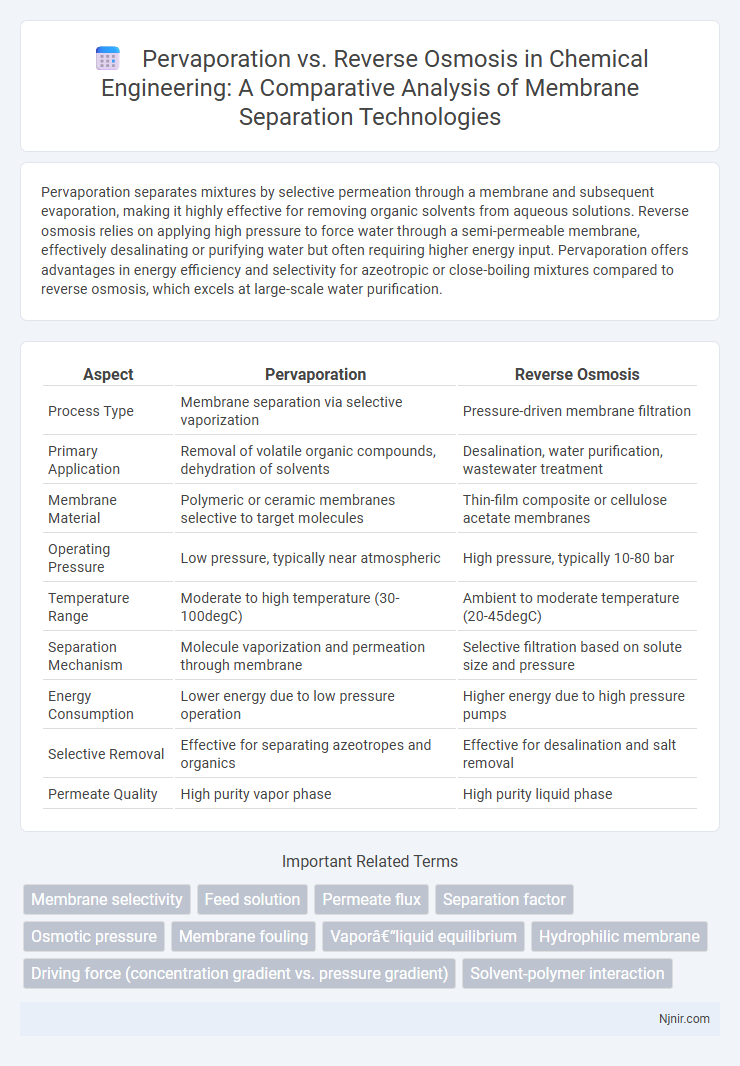
Table of Comparison
| Aspect | Pervaporation | Reverse Osmosis |
|---|---|---|
| Process Type | Membrane separation via selective vaporization | Pressure-driven membrane filtration |
| Primary Application | Removal of volatile organic compounds, dehydration of solvents | Desalination, water purification, wastewater treatment |
| Membrane Material | Polymeric or ceramic membranes selective to target molecules | Thin-film composite or cellulose acetate membranes |
| Operating Pressure | Low pressure, typically near atmospheric | High pressure, typically 10-80 bar |
| Temperature Range | Moderate to high temperature (30-100degC) | Ambient to moderate temperature (20-45degC) |
| Separation Mechanism | Molecule vaporization and permeation through membrane | Selective filtration based on solute size and pressure |
| Energy Consumption | Lower energy due to low pressure operation | Higher energy due to high pressure pumps |
| Selective Removal | Effective for separating azeotropes and organics | Effective for desalination and salt removal |
| Permeate Quality | High purity vapor phase | High purity liquid phase |
Introduction to Membrane Separation Technologies
Membrane separation technologies such as pervaporation and reverse osmosis are critical for efficient separation of mixtures based on selective permeability. Pervaporation utilizes a dense membrane to separate components by partial vaporization and diffusion, ideal for separating azeotropes and removing trace organics from liquids. Reverse osmosis relies on a semipermeable membrane to apply hydraulic pressure, effectively removing salts and contaminants from water for desalination and purification.
Fundamental Principles of Pervaporation
Pervaporation relies on selective membrane separation, where the feed mixture contacts one side of a dense, non-porous membrane that preferentially absorbs and diffuses specific components. The permeate then evaporates on the opposite side under reduced pressure or sweeping gas, enabling separation based on differences in volatility, solubility, and diffusion rates. Unlike reverse osmosis, which uses hydraulic pressure to separate solutes by size exclusion through porous membranes, pervaporation combines selective sorption and phase change for efficient separation of liquid mixtures.
Core Mechanisms of Reverse Osmosis
Reverse osmosis operates by applying pressure to force water through a semipermeable membrane, effectively separating contaminants based on molecular size and charge. The membrane's dense structure blocks larger solutes and dissolved salts while allowing purified water molecules to pass, ensuring high rejection rates of impurities. This pressure-driven mechanism contrasts with pervaporation, where selective evaporation through a membrane separates components based on volatility and permeability differences.
Key Differences in Operational Processes
Pervaporation separates components by selectively evaporating a liquid mixture through a membrane, relying on phase change and diffusion, whereas reverse osmosis forces liquid through a semi-permeable membrane using pressure, filtering out solutes without phase change. The pervaporation process involves vapor permeate collection under reduced pressure or sweeping gas, while reverse osmosis yields a liquid permeate with high solute rejection under high hydraulic pressure. Energy consumption in pervaporation is influenced by vaporization requirements, contrasting with the mechanical pressure-driven mechanism in reverse osmosis.
Membrane Materials and Configurations
Pervaporation membranes commonly utilize polymeric materials such as polydimethylsiloxane (PDMS) or polyvinyl alcohol (PVA) tailored for selective permeation, while reverse osmosis membranes primarily consist of thin-film composite (TFC) polyamide layers designed for high salt rejection. Configurations for pervaporation typically involve flat-sheet or tubular membranes arranged to maximize surface area and vapor collection, whereas reverse osmosis employs spiral-wound or hollow-fiber modules optimized for pressure-driven filtration. Material choice in pervaporation emphasizes chemical affinity and thermal stability, contrasting with reverse osmosis membranes where mechanical strength and chlorine resistance are critical for prolonged operational life.
Selectivity and Separation Efficiency
Pervaporation offers superior selectivity for separating azeotropic mixtures and organic solvents due to its membrane's molecular-level differentiation, achieving high separation efficiency in challenging separations. Reverse osmosis excels in desalination and removing inorganic salts with high rejection rates but struggles with separating mixtures of similar molecular sizes or volatile organic compounds. The separation efficiency of pervaporation is highly dependent on membrane material and operating conditions, often surpassing reverse osmosis in selectivity for complex liquid mixtures.
Applications in Chemical Engineering
Pervaporation is widely applied in chemical engineering for the separation of azeotropic mixtures and the dehydration of organic solvents, leveraging selective membrane permeation to enhance process efficiency. Reverse osmosis is predominantly used for desalination, wastewater treatment, and concentration of chemical solutions due to its high rejection rates of dissolved salts and impurities through semipermeable membranes. Both technologies optimize process intensification by reducing energy consumption and enabling continuous separation in various chemical manufacturing and environmental applications.
Energy Consumption and Environmental Impact
Pervaporation consumes significantly less energy than reverse osmosis due to its lower operating pressures and passive separation mechanism, making it more sustainable for separating azeotropic mixtures and removing organic solvents. Reverse osmosis relies on high-pressure pumps that require substantial electricity, leading to higher carbon footprints and operational costs, especially for large-scale desalination or wastewater treatment. Environmentally, pervaporation generates fewer brine wastes and chemical residues, reducing ecological harm compared to reverse osmosis, which produces concentrated brine streams that pose disposal challenges.
Cost Analysis and Economic Considerations
Pervaporation typically incurs higher initial capital costs due to specialized membrane materials and lower production scale compared to reverse osmosis, which benefits from mature technology and economies of scale. Operational expenses for pervaporation often include higher energy consumption per volume treated, while reverse osmosis systems are more energy-efficient but require costly maintenance for membrane fouling control. Overall, reverse osmosis tends to offer lower total cost of ownership for large-scale water treatment, whereas pervaporation may be economically justified for niche applications requiring selective separation or solvent recovery.
Future Trends and Technological Advances
Future trends in pervaporation and reverse osmosis emphasize enhanced membrane materials like graphene and mixed matrix membranes to improve selectivity and flux. Integration of hybrid systems combining pervaporation with reverse osmosis maximizes energy efficiency and contaminant removal in water treatment and chemical separations. Advances in nanotechnology and AI-driven process optimization are driving scalable, cost-effective solutions for industrial applications and environmental sustainability.
Membrane selectivity
Pervaporation membranes exhibit higher selectivity for separating azeotropic or close-boiling mixtures by selectively permeating specific components, whereas reverse osmosis membranes primarily rely on size exclusion and charge repulsion to separate solutes from solvents with moderate selectivity.
Feed solution
Pervaporation efficiently separates mixtures with organic components or azeotropes by selectively vaporizing the feed solution, while reverse osmosis primarily targets the removal of dissolved salts and contaminants from aqueous feed solutions through a pressure-driven membrane process.
Permeate flux
Pervaporation typically achieves lower permeate flux compared to reverse osmosis due to its membrane selectivity and operating conditions, which prioritize selective separation over volumetric throughput.
Separation factor
Pervaporation achieves higher separation factors for organic mixtures compared to reverse osmosis, which is more effective for desalination and inorganic salt removal.
Osmotic pressure
Pervaporation separates mixtures through selective membrane permeability without relying on osmotic pressure, whereas reverse osmosis uses high osmotic pressure gradients to drive solvent flow across a semipermeable membrane.
Membrane fouling
Pervaporation membranes experience less fouling compared to reverse osmosis membranes due to their selective permeation of volatile compounds, leading to longer operational stability and lower cleaning frequency.
Vapor–liquid equilibrium
Pervaporation exploits vapor-liquid equilibrium differences to separate components by selective vapor permeation through membranes, whereas reverse osmosis relies on pressure-driven liquid-phase separation without phase change.
Hydrophilic membrane
Hydrophilic membranes in pervaporation selectively separate water from organic solvents with higher separation efficiency and lower energy consumption compared to hydrophilic membranes used in reverse osmosis for desalination.
Driving force (concentration gradient vs. pressure gradient)
Pervaporation uses a concentration gradient as the driving force to separate components through a selective membrane, whereas reverse osmosis relies on a pressure gradient to force solvent molecules across a semi-permeable membrane.
Solvent-polymer interaction
Pervaporation leverages selective solvent-polymer interactions to separate mixtures based on differential sorption and diffusion, whereas reverse osmosis primarily relies on size exclusion and pressure-driven membrane permeability.
pervaporation vs reverse osmosis Infographic
 njnir.com
njnir.com