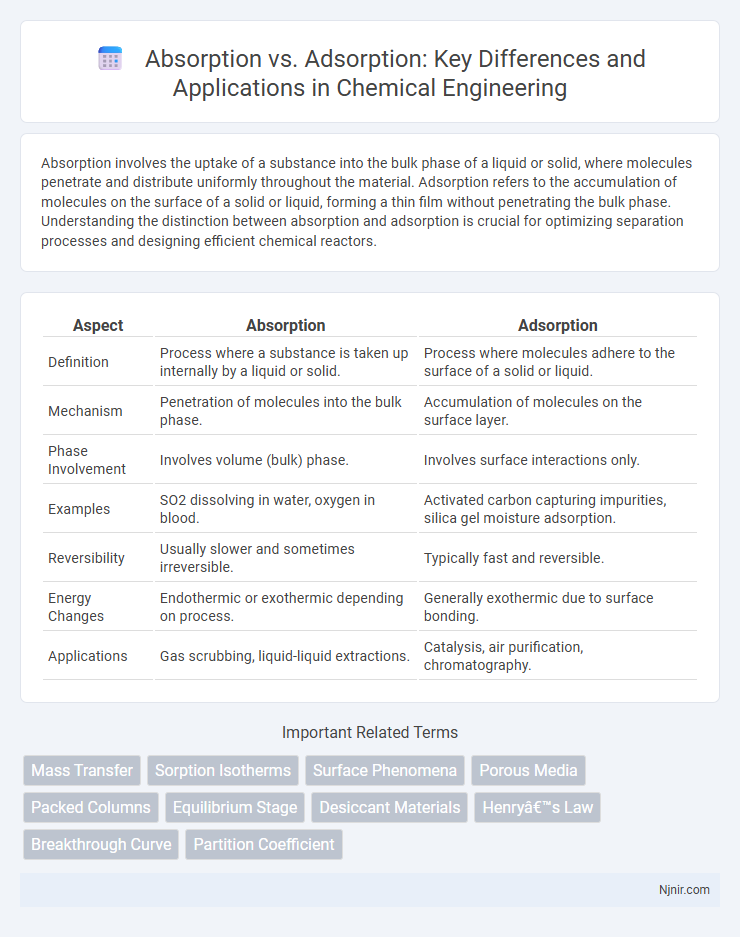
Absorption involves the uptake of a substance into the bulk phase of a liquid or solid, where molecules penetrate and distribute uniformly throughout the material. Adsorption refers to the accumulation of molecules on the surface of a solid or liquid, forming a thin film without penetrating the bulk phase. Understanding the distinction between absorption and adsorption is crucial for optimizing separation processes and designing efficient chemical reactors.
Table of Comparison
| Aspect | Absorption | Adsorption |
|---|---|---|
| Definition | Process where a substance is taken up internally by a liquid or solid. | Process where molecules adhere to the surface of a solid or liquid. |
| Mechanism | Penetration of molecules into the bulk phase. | Accumulation of molecules on the surface layer. |
| Phase Involvement | Involves volume (bulk) phase. | Involves surface interactions only. |
| Examples | SO2 dissolving in water, oxygen in blood. | Activated carbon capturing impurities, silica gel moisture adsorption. |
| Reversibility | Usually slower and sometimes irreversible. | Typically fast and reversible. |
| Energy Changes | Endothermic or exothermic depending on process. | Generally exothermic due to surface bonding. |
| Applications | Gas scrubbing, liquid-liquid extractions. | Catalysis, air purification, chromatography. |
Introduction to Absorption and Adsorption
Absorption involves the incorporation of substances into the bulk phase of a material, often seen in processes like gas absorption into liquids or solids. Adsorption, by contrast, is the accumulation of molecules on the surface of a solid or liquid, driven by surface energy interactions. Both phenomena are critical in fields such as catalysis, environmental engineering, and material science due to their role in separating and capturing substances.
Fundamental Principles of Absorption
Absorption involves the process where one substance penetrates into the bulk or interior of another, typically a solid or liquid, resulting in a homogeneous mixture at the molecular or atomic level. This phenomenon is governed by diffusion principles, driven by concentration gradients and thermodynamic compatibility between the absorbate and absorbent. Key parameters such as absorption capacity, rate, and equilibrium play crucial roles in industrial applications like gas scrubbing, liquid-liquid extraction, and chromatographic separations.
Core Concepts of Adsorption
Adsorption involves the accumulation of molecules on a solid or liquid surface, forming a molecular or atomic film, whereas absorption entails the entire volume of the material taking in the substance. The core concepts of adsorption include the formation of a surface layer through physical or chemical interactions, the distinction between physisorption involving weak van der Waals forces and chemisorption involving stronger chemical bonds, and the dependency on surface area and porosity of the adsorbent material. Adsorption capacity and kinetics are influenced by factors such as temperature, pressure, and the nature of the adsorbate and adsorbent, making it critical in applications like catalysis, water purification, and gas separation.
Key Differences Between Absorption and Adsorption
Absorption involves the uniform penetration of molecules into the bulk phase of a solid or liquid, whereas adsorption is the accumulation of molecules on the surface of a solid or liquid. Absorption affects the entire volume of the material, including solutes dissolving in solvents, while adsorption is limited to surface interactions involving active sites or pores. The process of absorption is generally slower and often reversible, in contrast to adsorption, which tends to be faster and can be either physical or chemical in nature depending on the bonding forces.
Types of Absorption Processes in Chemical Engineering
Absorption in chemical engineering involves the uptake of one substance into the bulk phase of another, typically a gas into a liquid or solid, categorized mainly into physical absorption and chemical absorption. Physical absorption relies on solubility and diffusion without chemical reaction, used in processes like gas scrubbing with water or organic solvents, while chemical absorption involves reactive solvents forming new compounds, crucial in removing acidic gases like CO2 or H2S from industrial streams. Understanding these types enables the design of efficient gas-liquid contactors and selection of appropriate solvents for separation and pollution control applications.
Classification of Adsorption Mechanisms
Adsorption mechanisms are primarily classified into physisorption and chemisorption based on the nature of the interactions between adsorbate and adsorbent. Physisorption involves weak van der Waals forces, which are generally reversible and characterized by low heat of adsorption, whereas chemisorption entails the formation of strong chemical bonds, often leading to irreversible adsorption with higher heat release. Factors such as surface energy, temperature, and pressure influence the dominance of each adsorption type in industrial and environmental applications.
Factors Affecting Absorption Efficiency
Absorption efficiency is influenced by factors such as temperature, pressure, and the surface area of the absorbent material. Higher temperatures can decrease gas solubility in liquids, reducing absorption, while increased pressure enhances gas solubility, improving absorption rates. The nature of the absorbent, including porosity and chemical affinity for the solute, also plays a critical role in determining overall absorption effectiveness.
Critical Parameters Impacting Adsorption
Critical parameters impacting adsorption include surface area, pore size distribution, temperature, pressure, and adsorbate concentration. High surface area and optimal pore sizes enhance adsorption capacity by providing more active sites for adsorbate molecules. Thermodynamic factors like temperature and pressure influence adsorption equilibria and kinetics, determining the efficiency and selectivity of the adsorption process.
Applications of Absorption in Industry
Absorption plays a crucial role in industries such as chemical manufacturing, environmental engineering, and petroleum refining, where it is used to remove gases like sulfur dioxide from emissions or to capture carbon dioxide for climate control. The process enables efficient separation and purification of liquids and gases, enhancing product quality in pharmaceutical and food processing sectors. Industrial applications also include solvent recovery and wastewater treatment, where absorption techniques optimize operational costs and environmental compliance.
Industrial Uses of Adsorption Techniques
Adsorption techniques play a crucial role in industrial applications such as gas purification, wastewater treatment, and catalyst recovery by selectively trapping contaminants on solid surfaces. Activated carbon, zeolites, and silica gels are widely employed as adsorbents due to their high surface area and affinity for specific molecules. This process enhances efficiency in air filtration, petrochemical refining, and removal of volatile organic compounds (VOCs), supporting sustainable and cost-effective manufacturing operations.
Mass Transfer
Mass transfer in absorption involves the bulk uptake of molecules into a liquid or solid phase, while adsorption entails the accumulation of molecules on the surface of a solid or liquid without bulk penetration.
Sorption Isotherms
Sorption isotherms characterize the relationship between the amount of substance adsorbed or absorbed and its pressure or concentration, with adsorption involving surface attachment and absorption involving bulk phase integration.
Surface Phenomena
Absorption involves the bulk uptake of substances into a material, whereas adsorption specifically refers to the accumulation of molecules only on the surface, highlighting distinct surface phenomena in chemical and physical processes.
Porous Media
Adsorption in porous media involves the accumulation of molecules on the surface of pores, while absorption entails the penetration and distribution of substances throughout the entire porous structure.
Packed Columns
Packed columns enhance mass transfer efficiency by promoting adsorption on solid surfaces, whereas absorption involves the bulk uptake of gases or vapors into a liquid phase within the column.
Equilibrium Stage
At the equilibrium stage, absorption involves the uniform distribution of molecules throughout the bulk phase, while adsorption occurs when molecules accumulate and adhere specifically at the interface or surface.
Desiccant Materials
Desiccant materials rely on adsorption to trap moisture on their surface, offering faster and more reversible drying compared to absorption, which involves moisture permeating into the bulk of the material.
Henry’s Law
Henry's Law quantifies gas absorption as the proportionality between gas solubility in a liquid and its partial pressure, distinct from adsorption which involves gas molecules adhering to solid surfaces.
Breakthrough Curve
The breakthrough curve in adsorption illustrates the point at which a contaminant first appears in the effluent, highlighting the adsorbent's capacity and saturation dynamics compared to absorption processes.
Partition Coefficient
The partition coefficient quantifies the distribution of a substance between absorption, where it penetrates the bulk phase, and adsorption, where it adheres to a surface.
Absorption vs Adsorption Infographic
 njnir.com
njnir.com