Biomass gasification converts organic materials into syngas through partial oxidation at high temperatures, enabling efficient energy production and reducing waste. Pyrolysis thermally decomposes biomass in an oxygen-free environment to produce bio-oil, char, and syngas with distinct chemical properties suitable for various applications. Gasification primarily emphasizes syngas generation for fuel and power, while pyrolysis offers more versatile outputs for energy and material uses.
Table of Comparison
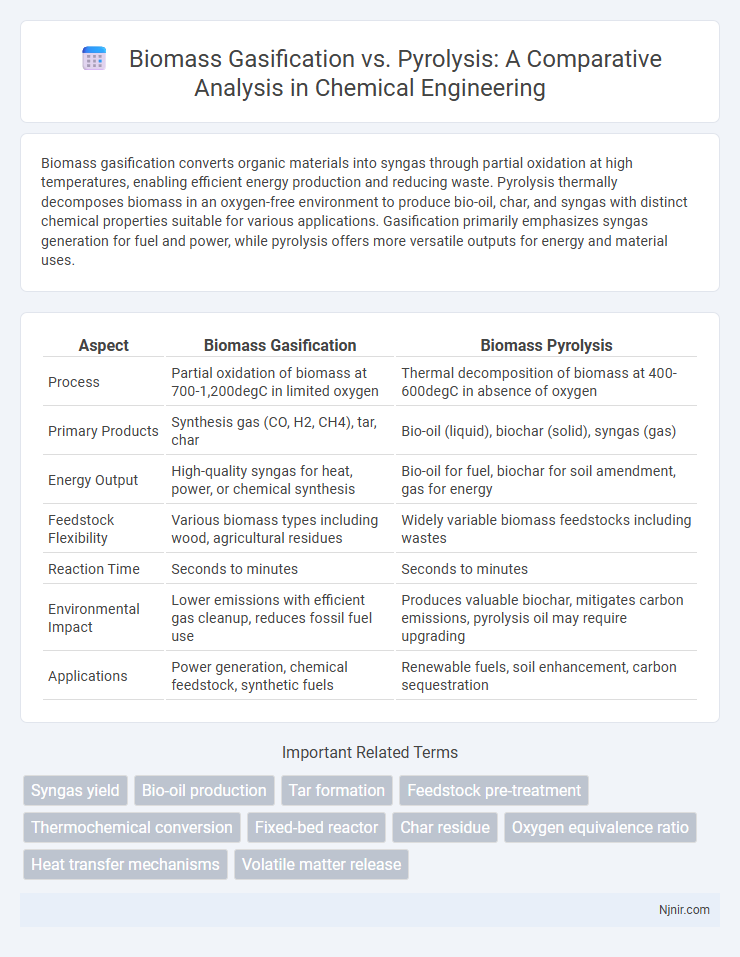
| Aspect | Biomass Gasification | Biomass Pyrolysis |
|---|---|---|
| Process | Partial oxidation of biomass at 700-1,200degC in limited oxygen | Thermal decomposition of biomass at 400-600degC in absence of oxygen |
| Primary Products | Synthesis gas (CO, H2, CH4), tar, char | Bio-oil (liquid), biochar (solid), syngas (gas) |
| Energy Output | High-quality syngas for heat, power, or chemical synthesis | Bio-oil for fuel, biochar for soil amendment, gas for energy |
| Feedstock Flexibility | Various biomass types including wood, agricultural residues | Widely variable biomass feedstocks including wastes |
| Reaction Time | Seconds to minutes | Seconds to minutes |
| Environmental Impact | Lower emissions with efficient gas cleanup, reduces fossil fuel use | Produces valuable biochar, mitigates carbon emissions, pyrolysis oil may require upgrading |
| Applications | Power generation, chemical feedstock, synthetic fuels | Renewable fuels, soil enhancement, carbon sequestration |
Introduction to Biomass Conversion Technologies
Biomass gasification converts organic materials into syngas through partial oxidation at high temperatures, enabling efficient energy production and chemical synthesis. Pyrolysis thermally decomposes biomass in the absence of oxygen, producing bio-oil, char, and gases, which offer versatile applications in fuel and material industries. Both technologies play pivotal roles in sustainable biomass conversion by transforming renewable feedstocks into valuable energy carriers and chemicals.
Fundamental Principles of Gasification
Biomass gasification involves the partial oxidation of organic material at high temperatures (700-1,000degC) in a controlled oxygen environment, producing syngas composed mainly of carbon monoxide, hydrogen, and methane. Unlike pyrolysis, which thermally decomposes biomass in the absence of oxygen to yield bio-oil, char, and syngas, gasification aims for a combustible gas mixture through gas-phase reactions including oxidation, reduction, and reforming. The fundamental principles of gasification emphasize controlling temperature, oxygen supply, and residence time to optimize syngas quality and energy efficiency.
Overview of Pyrolysis Processes
Pyrolysis processes thermochemically decompose biomass in an oxygen-limited environment, producing biochar, bio-oil, and syngas with varying yields depending on temperature and heating rates. Slow pyrolysis emphasizes char production through low heating rates and longer residence times, while fast pyrolysis maximizes bio-oil yield with rapid heating and short vapor residence times. This versatility in pyrolysis enables tailored conversion pathways for diverse bioenergy and biochemical applications compared to biomass gasification, which primarily focuses on syngas generation through partial oxidation.
Feedstock Suitability for Gasification vs. Pyrolysis
Biomass gasification is highly suitable for a wide range of feedstocks, including agricultural residues, wood chips, and energy crops, because it requires feedstocks with low moisture content and moderate particle size to ensure efficient conversion into syngas. Pyrolysis, on the other hand, can process more diverse feedstocks, including wet biomass and various organic wastes, due to its thermal decomposition at lower temperatures in the absence of oxygen, producing bio-oil, char, and syngas. The selection between gasification and pyrolysis feedstocks depends on moisture tolerance, particle size, and desired end-products for optimal energy recovery and resource utilization.
Reaction Mechanisms and Operating Conditions
Biomass gasification involves partial oxidation of biomass at high temperatures (700-1,000degC) with limited oxygen or steam, producing syngas composed mainly of CO, H2, and CH4 through complex reactions like carbon oxidation, water-gas shift, and methane reforming. Pyrolysis operates in oxygen-free environments at lower temperatures (400-600degC), thermally decomposing biomass into bio-oil, char, and non-condensable gases via bond cleavage and secondary cracking reactions. Gasification requires precise control of equivalence ratio and temperature to optimize syngas yield, while pyrolysis parameters such as heating rate, residence time, and temperature dictate the product distribution and quality.
Product Yields and Composition Comparison
Biomass gasification typically produces a higher yield of syngas composed mainly of hydrogen, carbon monoxide, and methane, making it suitable for energy applications. In contrast, pyrolysis generates a larger fraction of bio-oil and char, with bio-oil rich in organic compounds and char containing significant carbon content useful for soil amendment. Gasification favors gaseous fuel production, while pyrolysis offers diverse product streams with distinct chemical compositions tailored for fuels, chemicals, or soil enhancement.
Energy Efficiency and Process Integration
Biomass gasification typically achieves higher energy efficiency by converting organic materials into syngas with efficient heat integration, reaching thermal efficiencies of 70-85%. Pyrolysis, while producing valuable bio-oil and char, generally exhibits lower overall energy efficiency around 50-70%, due to complex heat management and product upgrading requirements. Integration of gasification into combined heat and power (CHP) systems enhances process efficiency, whereas pyrolysis benefits from integration with bio-refinery setups for maximal resource utilization.
Environmental Impacts and Emissions
Biomass gasification produces syngas with lower tar content and reduced particulate emissions compared to pyrolysis, which often emits higher levels of volatile organic compounds and polycyclic aromatic hydrocarbons. Gasification's partial oxidation process results in lower greenhouse gas emissions, particularly methane and carbon monoxide, whereas pyrolysis creates biochar and bio-oil but can lead to increased black carbon and non-methane hydrocarbons. Overall, gasification offers a cleaner energy conversion pathway with improved air quality benefits and reduced environmental pollution relative to pyrolysis.
Technological Challenges and Scale-Up
Biomass gasification faces technological challenges such as tar formation, feedstock variability, and the need for robust syngas cleaning systems to enable efficient scale-up. Pyrolysis encounters difficulties in controlling process parameters to optimize product yield and quality, as well as integrating bio-oil upgrading facilities for commercial viability. Scaling up both technologies requires advances in reactor design, process control, and feedstock logistics to achieve consistent operation and economic competitiveness in industrial applications.
Future Trends in Biomass Thermochemical Conversion
Future trends in biomass thermochemical conversion emphasize the integration of advanced sensors and AI to optimize gasification and pyrolysis processes, enhancing energy efficiency and product yield. Innovations in catalyst development and reactor design aim to reduce tar formation during gasification, while pyrolysis advancements focus on producing high-value biochar and bio-oil with tailored properties for soil amendment and renewable fuels. Scaling up commercial applications and incorporating hybrid systems combining gasification and pyrolysis are projected to accelerate sustainable bioenergy production and carbon neutrality goals.
Syngas yield
Biomass gasification typically produces a higher syngas yield with greater hydrogen and carbon monoxide content compared to pyrolysis, which mainly generates bio-oil and char alongside lower volumes of syngas.
Bio-oil production
Biomass pyrolysis produces higher yields of energy-dense bio-oil compared to biomass gasification, which primarily generates syngas with lower bio-oil output.
Tar formation
Biomass gasification generates higher tar formation compared to pyrolysis due to incomplete thermal decomposition of organic compounds at lower temperatures and varied oxidation environments.
Feedstock pre-treatment
Feedstock pre-treatment for biomass gasification typically involves drying and size reduction to enhance gas yield and tar reduction, whereas pyrolysis pre-treatment prioritizes uniform particle size and moisture control to optimize bio-oil production and carbon char quality.
Thermochemical conversion
Biomass gasification converts organic material into syngas through partial oxidation at high temperatures, while pyrolysis thermally decomposes biomass in the absence of oxygen to produce bio-oil, char, and syngas, both serving as key thermochemical conversion methods for renewable energy production.
Fixed-bed reactor
Fixed-bed reactors in biomass gasification efficiently convert solid biomass into syngas with high carbon conversion, while pyrolysis in fixed-bed systems primarily produces bio-oil and char through thermal decomposition under limited oxygen conditions.
Char residue
Biomass gasification produces a lower amount of char residue with higher carbon content compared to pyrolysis, making it more efficient for syngas production while pyrolysis yields higher char for soil amendment and activated carbon applications.
Oxygen equivalence ratio
Biomass gasification operates at an oxygen equivalence ratio typically between 0.2 and 0.4 to ensure partial oxidation, whereas pyrolysis occurs at near-zero oxygen equivalence ratios to favor thermal decomposition without combustion.
Heat transfer mechanisms
Biomass gasification primarily utilizes convective and radiative heat transfer to sustain high-temperature partial oxidation, while pyrolysis relies on conductive and convective heat transfer to thermally decompose biomass in an oxygen-limited environment.
Volatile matter release
Biomass gasification releases a higher proportion of volatile matter rapidly at elevated temperatures, while pyrolysis produces volatiles more gradually, emphasizing thermal decomposition under limited oxygen conditions.
biomass gasification vs pyrolysis Infographic
 njnir.com
njnir.com