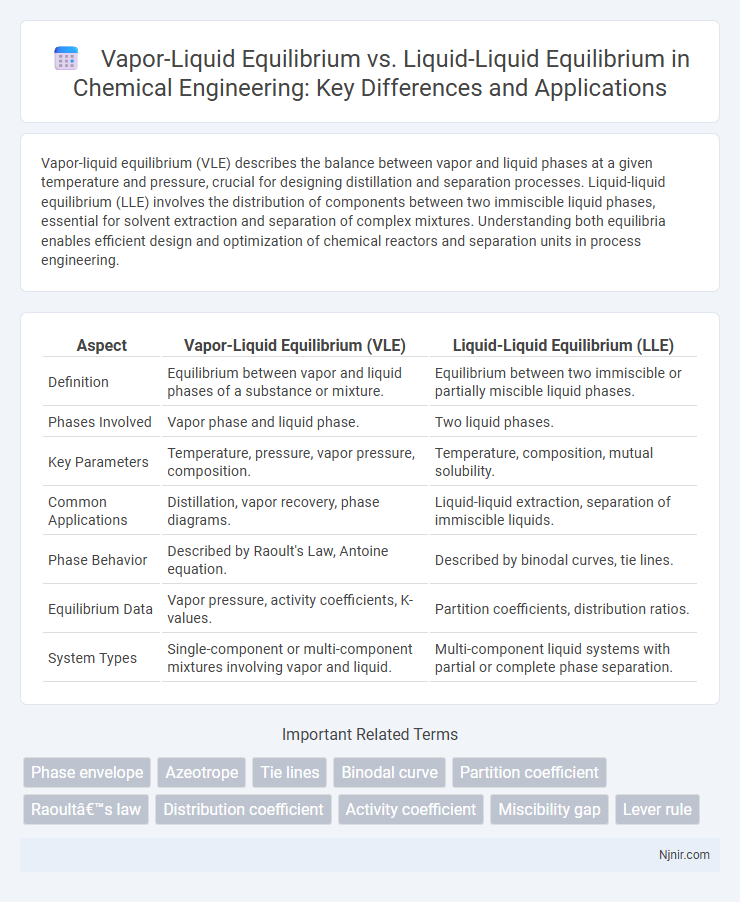
Vapor-liquid equilibrium (VLE) describes the balance between vapor and liquid phases at a given temperature and pressure, crucial for designing distillation and separation processes. Liquid-liquid equilibrium (LLE) involves the distribution of components between two immiscible liquid phases, essential for solvent extraction and separation of complex mixtures. Understanding both equilibria enables efficient design and optimization of chemical reactors and separation units in process engineering.
Table of Comparison
| Aspect | Vapor-Liquid Equilibrium (VLE) | Liquid-Liquid Equilibrium (LLE) |
|---|---|---|
| Definition | Equilibrium between vapor and liquid phases of a substance or mixture. | Equilibrium between two immiscible or partially miscible liquid phases. |
| Phases Involved | Vapor phase and liquid phase. | Two liquid phases. |
| Key Parameters | Temperature, pressure, vapor pressure, composition. | Temperature, composition, mutual solubility. |
| Common Applications | Distillation, vapor recovery, phase diagrams. | Liquid-liquid extraction, separation of immiscible liquids. |
| Phase Behavior | Described by Raoult's Law, Antoine equation. | Described by binodal curves, tie lines. |
| Equilibrium Data | Vapor pressure, activity coefficients, K-values. | Partition coefficients, distribution ratios. |
| System Types | Single-component or multi-component mixtures involving vapor and liquid. | Multi-component liquid systems with partial or complete phase separation. |
Introduction to Phase Equilibria in Chemical Engineering
Vapor-liquid equilibrium (VLE) and liquid-liquid equilibrium (LLE) are fundamental concepts in phase equilibria critical to chemical engineering, governing the distribution of components between different phases at equilibrium. VLE describes the balance between vapor and liquid phases, influencing processes like distillation and evaporation through temperature, pressure, and composition relationships. LLE, by contrast, involves the coexistence of two immiscible liquid phases, crucial for extraction and solvent recovery, where phase compositions depend on interactions, temperature, and pressure.
Fundamentals of Vapor-Liquid Equilibrium (VLE)
Vapor-liquid equilibrium (VLE) occurs when a liquid and its vapor phase coexist at a specific temperature and pressure, with the chemical potential of each component equal in both phases, enabling predictable phase compositions. VLE is governed by Raoult's law, Dalton's law, and modified activity coefficient models to describe non-ideal mixtures, critical for designing distillation and separation processes. In contrast, liquid-liquid equilibrium involves two immiscible or partially miscible liquid phases with distinct compositions, driven by mutual solubility and interfacial tension instead of vapor pressure differences.
Fundamentals of Liquid-Liquid Equilibrium (LLE)
Liquid-liquid equilibrium (LLE) involves the coexistence of two immiscible or partially miscible liquid phases at equilibrium, characterized by the distribution of components based on their chemical potentials and activity coefficients in each phase. Unlike vapor-liquid equilibrium (VLE), which involves phase transition between liquid and vapor, LLE focuses on phase separation within liquids due to differences in molecular interactions and solubility parameters. Understanding the Gibbs phase rule, tie lines, and binodal curves is fundamental to modeling LLE, enabling accurate prediction of composition and phase behavior in solvent extraction, chemical separations, and formulation processes.
Thermodynamic Principles Governing VLE and LLE
Vapor-liquid equilibrium (VLE) and liquid-liquid equilibrium (LLE) are governed by phase equilibrium thermodynamics, where chemical potential equality drives phase coexistence. In VLE, the equilibrium is established by balancing the fugacities of components in vapor and liquid phases, governed by Raoult's and Dalton's laws with non-idealities captured through activity coefficients or equations of state. LLE depends on the equality of component chemical potentials between immiscible liquid phases, influenced by Gibbs free energy of mixing, activity coefficients, and phase stability criteria under temperature and pressure constraints.
Key Differences Between VLE and LLE Systems
Vapor-liquid equilibrium (VLE) involves the coexistence of vapor and liquid phases with species distributed based on volatility differences, governed by temperature, pressure, and composition. Liquid-liquid equilibrium (LLE) describes the coexistence of two immiscible or partially miscible liquid phases separated due to differences in solubility and molecular interactions at specific conditions. Key differences include phase types involved, driving forces (volatility in VLE versus solubility in LLE), and applications such as distillation for VLE and solvent extraction for LLE.
Mathematical Models for VLE and LLE Calculations
Mathematical models for Vapor-Liquid Equilibrium (VLE) calculations commonly utilize activity coefficient models such as Wilson, NRTL, and UNIQUAC to represent non-ideal liquid phase behavior, along with equations of state like Peng-Robinson and Soave-Redlich-Kwong for vapor phase properties. Liquid-Liquid Equilibrium (LLE) models focus primarily on phase-split calculations using activity coefficient models to describe mutual solubility and phase compositions, often employing modified versions of NRTL and UNIQUAC for liquid phase non-ideality. Both VLE and LLE calculations rely on rigorous iterative numerical methods to solve nonlinear equations governing phase equilibria, optimizing parameters to fit experimental data for accurate phase diagram predictions.
Experimental Methods for Determining VLE and LLE Data
Experimental methods for determining vapor-liquid equilibrium (VLE) data commonly involve isobaric or isothermal VLE measurements using apparatuses such as ebulliometers, recirculating stills, or cell-type equilibrium columns that allow direct sampling of vapor and liquid phases. Liquid-liquid equilibrium (LLE) data are typically obtained through cloud point titration, analytical centrifugation, or decantation techniques combined with chemical analysis methods like gas chromatography or refractive index measurements to determine phase compositions accurately. Both VLE and LLE experiments require precise temperature and pressure control to ensure equilibrium conditions, with VLE focusing on vapor-liquid phase coexistence and LLE on the compositions of immiscible liquid phases.
Industrial Applications of VLE vs LLE
Vapor-liquid equilibrium (VLE) is critical in industrial distillation processes where separation of components based on volatility differences occurs, such as in petroleum refining and chemical manufacturing. Liquid-liquid equilibrium (LLE) finds application in solvent extraction and separation of immiscible liquid mixtures, commonly used in pharmaceutical production and wastewater treatment. VLE processes optimize energy-efficient phase separation, while LLE focuses on selective solute transfer between two liquid phases for enhanced purity.
Challenges in Simulating VLE and LLE in Chemical Processes
Simulating vapor-liquid equilibrium (VLE) presents challenges in accurately modeling phase behavior under varying temperature and pressure, requiring precise thermodynamic properties and handling non-ideal mixtures. Liquid-liquid equilibrium (LLE) simulations face difficulties due to complex mutual solubilities, presence of multiple liquid phases, and sharp composition gradients, demanding robust algorithms for phase splitting and activity coefficient calculations. Both VLE and LLE simulations must address issues such as convergence stability, accurate phase boundary predictions, and incorporation of experimental data for model validation.
Future Trends in Phase Equilibrium Research
Future trends in phase equilibrium research increasingly emphasize advanced computational methods and machine learning algorithms to predict vapor-liquid and liquid-liquid equilibrium with higher accuracy. Integration of molecular simulations and big data analytics enhances understanding of complex multicomponent systems, facilitating the design of sustainable chemical processes. Emerging applications in energy storage, carbon capture, and bioseparations drive innovation in phase equilibrium modeling and experimental techniques.
Phase envelope
Phase envelopes for vapor-liquid equilibrium illustrate temperature-pressure boundaries where vapor and liquid phases coexist, whereas liquid-liquid equilibrium phase envelopes delineate compositions and conditions under which two immiscible liquid phases coexist.
Azeotrope
Azeotropes in vapor-liquid equilibrium represent constant-boiling mixtures that cannot be separated by simple distillation, whereas in liquid-liquid equilibrium, azeotrope behavior influences phase separation due to immiscibility and unique composition ratios.
Tie lines
Tie lines in vapor-liquid equilibrium connect compositions of coexisting vapor and liquid phases, while in liquid-liquid equilibrium they link the compositions of immiscible liquid phases in equilibrium.
Binodal curve
The binodal curve delineates the phase boundaries in liquid-liquid equilibrium, marking the compositions at which a homogeneous mixture separates into two distinct liquid phases, whereas vapor-liquid equilibrium involves equilibrium compositions defined by vapor pressure and temperature without a binodal curve concept.
Partition coefficient
Partition coefficient quantifies component distribution in vapor-liquid equilibrium as the ratio of vapor to liquid concentrations, whereas in liquid-liquid equilibrium it represents solute distribution ratio between two immiscible liquid phases.
Raoult’s law
Raoult's law describes vapor-liquid equilibrium by relating the partial vapor pressure of each component to its mole fraction in the liquid phase, while liquid-liquid equilibrium involves the distribution of components between two immiscible liquid phases without significant vapor pressure considerations.
Distribution coefficient
Vapor-liquid equilibrium involves phase distribution of a component between vapor and liquid phases with a distribution coefficient defined by its vapor-liquid partition ratio, whereas liquid-liquid equilibrium describes solute distribution between two immiscible liquids characterized by a distribution coefficient quantifying solute concentration ratios across both liquid phases.
Activity coefficient
Activity coefficients in vapor-liquid equilibrium quantify non-ideal interactions affecting phase composition, while in liquid-liquid equilibrium they determine the extent of mutual solubility and phase separation.
Miscibility gap
The miscibility gap in liquid-liquid equilibrium defines the composition range where two immiscible liquid phases coexist, contrasting with vapor-liquid equilibrium where phase separation occurs between vapor and a single liquid phase.
Lever rule
The Lever rule is primarily applied in liquid-liquid equilibrium to determine phase compositions and relative amounts, whereas in vapor-liquid equilibrium it is used less frequently because phase boundaries are defined by composition and temperature rather than lever-like linear segments.
Vapor-liquid equilibrium vs Liquid-liquid equilibrium Infographic
 njnir.com
njnir.com