Distillation separates components based on differences in boiling points, making it ideal for mixtures of volatile liquids, while extraction relies on solubility differences to transfer a solute from one liquid phase to another. The efficiency of distillation depends on precise temperature control and column design, whereas extraction effectiveness is influenced by the choice of solvent and phase equilibrium. Both techniques are fundamental in chemical engineering for purifying compounds, but their application depends on the physical and chemical properties of the substances involved.
Table of Comparison
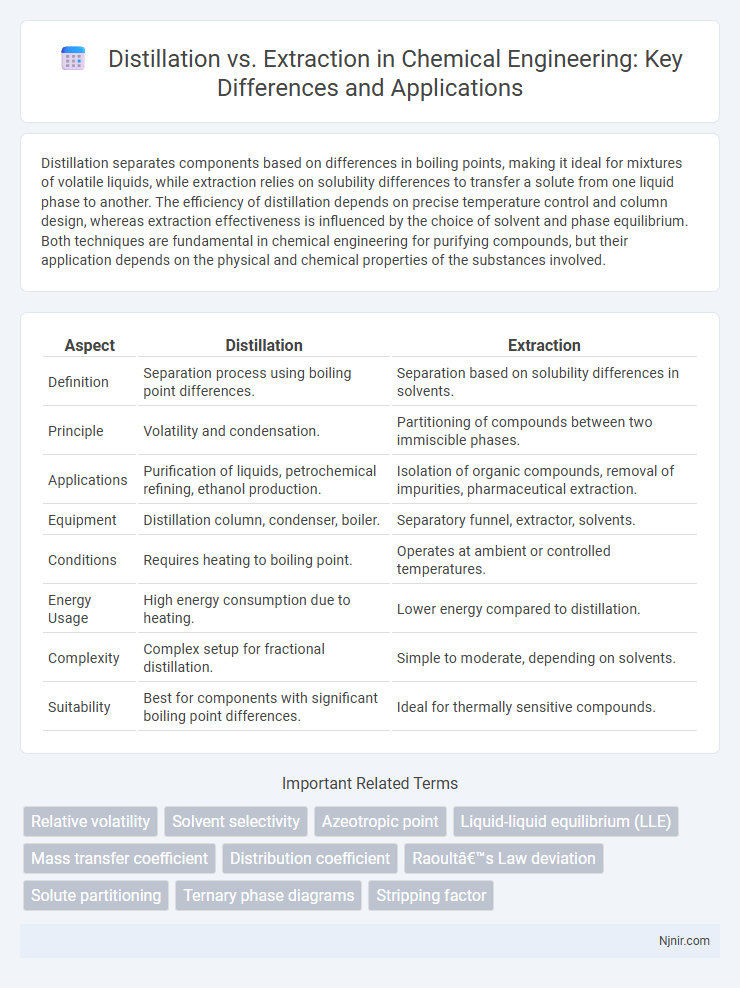
| Aspect | Distillation | Extraction |
|---|---|---|
| Definition | Separation process using boiling point differences. | Separation based on solubility differences in solvents. |
| Principle | Volatility and condensation. | Partitioning of compounds between two immiscible phases. |
| Applications | Purification of liquids, petrochemical refining, ethanol production. | Isolation of organic compounds, removal of impurities, pharmaceutical extraction. |
| Equipment | Distillation column, condenser, boiler. | Separatory funnel, extractor, solvents. |
| Conditions | Requires heating to boiling point. | Operates at ambient or controlled temperatures. |
| Energy Usage | High energy consumption due to heating. | Lower energy compared to distillation. |
| Complexity | Complex setup for fractional distillation. | Simple to moderate, depending on solvents. |
| Suitability | Best for components with significant boiling point differences. | Ideal for thermally sensitive compounds. |
Introduction to Separation Processes in Chemical Engineering
Distillation and extraction are fundamental separation processes in chemical engineering used to isolate components based on differences in volatility and solubility, respectively. Distillation exploits boiling point differences to separate liquid mixtures into individual components through vaporization and condensation. Extraction separates solutes from one liquid phase into another immiscible liquid phase based on differential solubility, commonly applied to remove impurities or recover valuable compounds.
Fundamentals of Distillation
Distillation relies on the principle of differences in boiling points to separate components in a mixture by vaporizing and subsequently condensing the more volatile substances. It involves phase changes between liquid and vapor phases, enabling purification based on volatility variations. The efficiency of distillation depends on factors like vapor-liquid equilibrium, column design, and reflux ratio, which optimize separation performance.
Principles of Extraction
Extraction relies on the principle of selectively dissolving target compounds from a mixture into a suitable solvent based on differences in solubility and polarity. This method preserves the integrity of heat-sensitive compounds by operating at lower temperatures compared to distillation, which depends on differences in boiling points to separate components. Efficient extraction requires careful selection of solvents to maximize yield and purity of the desired substances.
Distillation vs Extraction: Process Mechanisms
Distillation separates components based on differences in boiling points through vaporization and condensation cycles, efficiently isolating volatile compounds from mixtures. Extraction relies on solubility differences, using solvents to selectively dissolve and separate target substances from solid or liquid matrices. Both processes utilize distinct physical properties--volatility for distillation and solubility for extraction--to achieve separation in chemical, pharmaceutical, and food industries.
Equipment Used in Distillation and Extraction
Distillation equipment primarily includes a distillation flask, condenser, and receiving flask, designed to separate components based on boiling points through vaporization and condensation. Extraction apparatus often involves a separatory funnel or Soxhlet extractor, facilitating the transfer of solutes from one solvent to another by exploiting differences in solubility. Both processes employ specialized equipment to optimize the separation and purification of chemical mixtures.
Efficiency and Selectivity Comparison
Distillation exhibits high efficiency in separating components with significant boiling point differences but struggles with close-boiling mixtures, leading to lower selectivity. Extraction offers superior selectivity by targeting specific solubility differences between compounds, making it more effective for separating thermally sensitive or closely related substances. Efficiency in extraction depends heavily on solvent choice and phase equilibrium, often resulting in fewer energy requirements compared to the heat-intensive distillation process.
Energy Consumption and Economic Considerations
Distillation typically requires higher energy consumption due to the need for continuous heating and vaporization, making it less cost-effective for heat-sensitive or dilute mixtures. Extraction often consumes less energy as it operates at lower temperatures and can use solvents to selectively separate compounds, reducing operational costs. Economic considerations include initial equipment investment for extraction being higher but offset by lower energy bills, while distillation benefits from established technology but incurs substantial ongoing energy expenses.
Applications in Industry: Distillation vs Extraction
Distillation is widely applied in the petrochemical industry for separating crude oil into fractions such as gasoline, kerosene, and diesel, utilizing differences in boiling points. Extraction is pivotal in the pharmaceutical sector for isolating active compounds from plant materials using solvents tailored to target specific chemical properties. Both techniques are essential for purifying chemicals, with distillation excelling in volatility-based separation and extraction specializing in solubility-driven isolation processes.
Environmental and Safety Impacts
Distillation and extraction differ significantly in environmental and safety impacts, with distillation often requiring high energy input and generating greenhouse gas emissions due to heating processes. Extraction, particularly when using hazardous solvents, poses risks of chemical exposure, environmental contamination, and solvent disposal challenges. Implementing green solvents and energy-efficient distillation techniques can mitigate negative environmental and safety effects in both methods.
Choosing Between Distillation and Extraction
Choosing between distillation and extraction depends on the physical and chemical properties of the substances involved, such as boiling points and solubility. Distillation is ideal for separating components with significant boiling point differences, efficiently purifying volatile compounds. Extraction suits scenarios where components differ in polarity or solubility, enabling selective separation using appropriate solvents.
Relative volatility
Relative volatility measures the ease of separation in distillation by comparing component vapor pressures, whereas extraction relies on solubility differences without involving relative volatility.
Solvent selectivity
Solvent selectivity in distillation relies on differences in boiling points, while extraction depends on the solvent's ability to preferentially dissolve target compounds based on polarity and solubility.
Azeotropic point
Distillation separates mixtures based on boiling point differences but struggles with azeotropic points where vapor and liquid compositions match, while extraction bypasses phase change by dissolving components in a solvent to separate azeotropes effectively.
Liquid-liquid equilibrium (LLE)
Liquid-liquid equilibrium (LLE) is crucial for optimizing separation efficiency, where distillation relies on vapor-liquid equilibrium and extraction exploits immiscible liquid phases to achieve targeted component separation.
Mass transfer coefficient
The mass transfer coefficient in distillation is typically higher than in extraction due to phase change enhancing mass transfer rates between vapor and liquid phases.
Distribution coefficient
The distribution coefficient quantifies the equilibrium concentration ratio of a solute between two immiscible phases, critically influencing the efficiency of extraction compared to distillation, which relies on volatility differences rather than solute partitioning.
Raoult’s Law deviation
Distillation efficiency decreases in mixtures exhibiting Raoult's Law deviation, while extraction remains effective for separating non-ideal solutions with significant vapor-liquid equilibrium deviations.
Solute partitioning
Solute partitioning in distillation involves phase changes based on volatility differences between components, whereas in extraction it depends on differential solubility in immiscible liquid solvents.
Ternary phase diagrams
Ternary phase diagrams visually represent the equilibrium compositions in Distillation and Extraction, illustrating the different phase separations and component distributions critical for optimizing separation processes.
Stripping factor
Stripping factor in distillation measures the ratio of solvent to solute removal efficiency, while in extraction it quantifies the distribution coefficient influencing solute transfer between phases.
Distillation vs Extraction Infographic
 njnir.com
njnir.com