Nanofiltration membranes selectively remove divalent and larger monovalent ions, making them ideal for water softening and partial desalination, whereas ultrafiltration membranes primarily target suspended solids, bacteria, and large macromolecules. Nanofiltration operates with smaller pore sizes (approximately 1-10 nanometers) compared to ultrafiltration (around 10-100 nanometers), resulting in higher molecular weight cut-offs and increased rejection rates for organic compounds. The choice between nanofiltration and ultrafiltration depends on the specific separation requirements, such as ion removal efficiency versus particulate filtration.
Table of Comparison
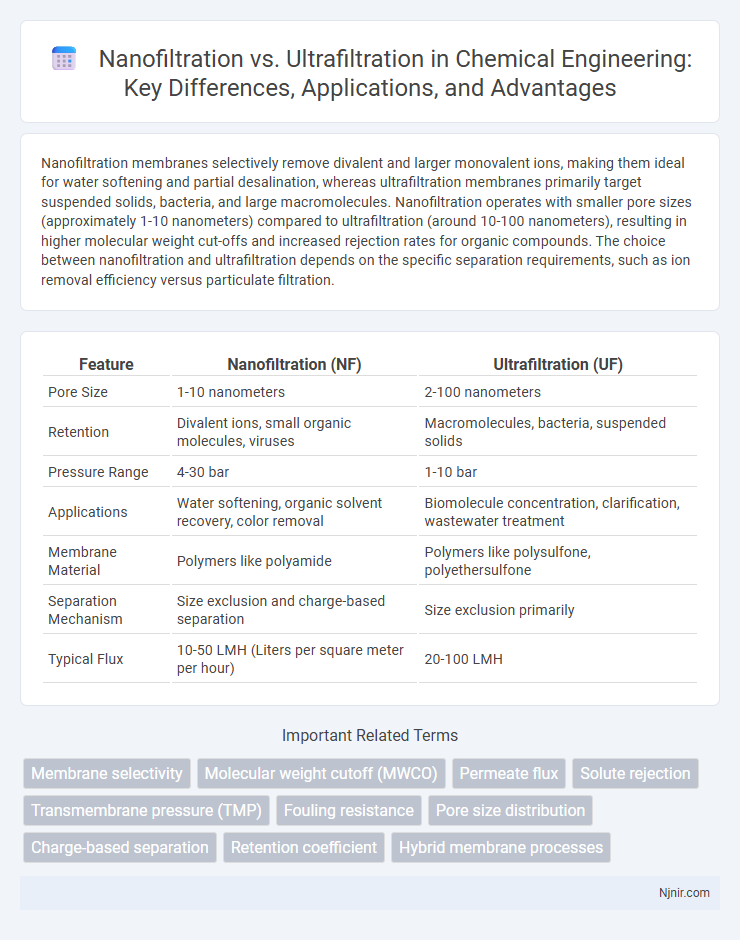
| Feature | Nanofiltration (NF) | Ultrafiltration (UF) |
|---|---|---|
| Pore Size | 1-10 nanometers | 2-100 nanometers |
| Retention | Divalent ions, small organic molecules, viruses | Macromolecules, bacteria, suspended solids |
| Pressure Range | 4-30 bar | 1-10 bar |
| Applications | Water softening, organic solvent recovery, color removal | Biomolecule concentration, clarification, wastewater treatment |
| Membrane Material | Polymers like polyamide | Polymers like polysulfone, polyethersulfone |
| Separation Mechanism | Size exclusion and charge-based separation | Size exclusion primarily |
| Typical Flux | 10-50 LMH (Liters per square meter per hour) | 20-100 LMH |
Introduction to Nanofiltration and Ultrafiltration
Nanofiltration (NF) is a membrane filtration process that targets particles in the 1-10 nanometer range, effectively removing divalent and larger monovalent ions, organic molecules, and pathogens while allowing smaller monovalent ions to pass through. Ultrafiltration (UF) utilizes membranes with pore sizes of approximately 0.01 to 0.1 microns to reject suspended solids, bacteria, viruses, and macromolecules such as proteins, without removing dissolved salts or low molecular weight solutes. Both NF and UF are essential in water treatment, food processing, and pharmaceutical industries, with NF providing partial desalination and UF focusing on particulate removal and molecular separation.
Fundamental Principles of Membrane Filtration
Nanofiltration operates on a principle that combines size exclusion and charge-based separation, targeting molecules in the 1-10 nanometer range, effectively removing divalent and larger monovalent ions. Ultrafiltration relies primarily on size exclusion with membrane pores sized between 1-100 nanometers, efficiently retaining macromolecules, proteins, and suspended solids while allowing smaller solutes to pass through. Both technologies utilize pressure-driven membrane processes but differ in pore size and separation mechanisms, influencing their applications in water treatment and product purification.
Membrane Material and Pore Size Comparison
Nanofiltration membranes typically utilize polyamide or thin-film composite materials, offering pore sizes ranging from 1 to 10 nanometers, ideal for selectively removing divalent salts and larger organic molecules. Ultrafiltration membranes are commonly made from materials like polysulfone, polyethersulfone, or cellulose acetate, with larger pore sizes between 5 to 100 nanometers, suitable for retaining macromolecules, viruses, and suspended solids. The smaller pore size and denser membrane structure in nanofiltration allow for higher rejection rates of specific solutes compared to ultrafiltration's broader filtration capabilities.
Mechanisms of Separation in Nanofiltration vs Ultrafiltration
Nanofiltration utilizes a combination of size exclusion and charge-based separation mechanisms to selectively filter out molecules typically in the 200-1000 Dalton range, effectively removing divalent and larger monovalent ions. Ultrafiltration relies primarily on size exclusion, with membrane pore sizes ranging from 1 to 100 nanometers, capturing larger particles such as proteins, bacteria, and suspended solids. The key distinction between nanofiltration and ultrafiltration lies in nanofiltration's ability to separate smaller solutes and ions through both steric hindrance and electrostatic interactions, whereas ultrafiltration is predominantly a physical barrier against macromolecules.
Performance Parameters: Flux, Selectivity, and Permeate Quality
Nanofiltration membranes typically exhibit higher selectivity for divalent and larger monovalent ions compared to ultrafiltration, which mainly targets macromolecules and suspended solids. Flux rates in ultrafiltration tend to be higher due to larger pore sizes, enabling greater water permeability but with lower rejection of smaller solutes. Permeate quality in nanofiltration surpasses ultrafiltration in removing dissolved salts and organics, resulting in cleaner water suitable for more demanding applications.
Applications in Chemical Engineering Industries
Nanofiltration membranes effectively separate divalent and larger monovalent ions, making them ideal for softening water and removing organic compounds in pharmaceutical and food processing industries. Ultrafiltration membranes retain macromolecules and suspended solids, widely applied in wastewater treatment and protein concentration processes within chemical manufacturing. Both technologies enhance resource recovery and process efficiency by tailoring separation based on molecular weight cut-off and pore size.
Removal of Contaminants and Molecular Weight Cut-off
Nanofiltration membranes typically remove contaminants with molecular weights around 200-1000 Daltons, effectively filtering out divalent and larger monovalent ions, organic molecules, and some pathogens. Ultrafiltration membranes have a higher molecular weight cut-off, generally between 1,000 and 100,000 Daltons, allowing them to retain larger particles like proteins, bacteria, and suspended solids while passing smaller solutes. The choice between nanofiltration and ultrafiltration depends on the specific treatment goals related to the size and type of contaminants targeted for removal.
Operational Challenges and Fouling Behavior
Nanofiltration membranes exhibit higher susceptibility to fouling due to their smaller pore size compared to ultrafiltration, leading to frequent cleaning requirements and operational downtime. Ultrafiltration membranes typically handle higher loads of suspended solids and organic matter with lower fouling rates, enhancing process stability in treating wastewater and industrial effluents. Operational challenges for nanofiltration include managing membrane compaction and scaling caused by divalent ions, while ultrafiltration systems face challenges primarily related to biofouling and particulate accumulation.
Economic Considerations and Energy Efficiency
Nanofiltration membranes generally offer higher operational costs due to tighter pore sizes requiring increased pressure, leading to greater energy consumption compared to ultrafiltration. Ultrafiltration membranes exhibit lower energy requirements and maintenance expenses because they operate under lower pressures, making them more cost-effective for large-scale water treatment. When evaluating economic considerations, nanofiltration provides better removal of divalent salts and organic compounds, but ultrafiltration's energy efficiency and reduced fouling result in lower overall lifecycle costs.
Future Trends in Membrane Filtration Technologies
Nanofiltration and ultrafiltration membranes are evolving with enhanced selectivity and permeability to address emerging contaminants and water scarcity challenges. Future trends include the integration of advanced nanomaterials, such as graphene oxide and carbon nanotubes, to improve fouling resistance and energy efficiency. Hybrid membrane systems combining nanofiltration and ultrafiltration processes are gaining traction for wastewater reuse and resource recovery in industrial and municipal applications.
Membrane selectivity
Nanofiltration membranes exhibit higher selectivity by effectively removing divalent ions and larger organic molecules, whereas ultrafiltration membranes primarily retain macromolecules like proteins and suspended solids due to their larger pore sizes.
Molecular weight cutoff (MWCO)
Nanofiltration membranes typically have a molecular weight cutoff (MWCO) between 200 and 1000 Daltons, effectively removing small organic molecules and divalent ions, whereas ultrafiltration membranes possess a higher MWCO range of 1,000 to 100,000 Daltons, filtering out larger macromolecules like proteins and suspended solids.
Permeate flux
Nanofiltration generally achieves higher permeate flux rates than ultrafiltration due to its finer membrane pore size and improved selectivity.
Solute rejection
Nanofiltration exhibits higher solute rejection rates for divalent and larger molecules compared to ultrafiltration, which primarily rejects suspended solids and macromolecules.
Transmembrane pressure (TMP)
Nanofiltration typically operates at higher transmembrane pressures (4-10 bar) compared to ultrafiltration (1-4 bar), enabling finer solute separation and improved filtration efficiency.
Fouling resistance
Nanofiltration membranes demonstrate higher fouling resistance than ultrafiltration membranes due to their tighter pore structure and enhanced rejection of organic and particulate matter.
Pore size distribution
Nanofiltration membranes typically have a pore size distribution ranging from 1 to 10 nanometers, enabling the selective removal of divalent ions and small organic molecules, whereas ultrafiltration membranes feature larger pores, usually between 10 and 100 nanometers, suited for filtering macromolecules and suspended solids.
Charge-based separation
Nanofiltration membranes utilize charge-based separation to selectively reject divalent and larger ions while allowing monovalent ions to pass, whereas ultrafiltration membranes primarily rely on size exclusion and have limited charge-based separation capabilities.
Retention coefficient
Nanofiltration typically exhibits a higher retention coefficient for divalent and larger molecules compared to ultrafiltration, which primarily retains macromolecules and particles based on size exclusion.
Hybrid membrane processes
Hybrid membrane processes combining nanofiltration and ultrafiltration enhance water treatment efficiency by improving contaminant removal, flux rates, and fouling resistance through synergistic separation mechanisms.
nanofiltration vs ultrafiltration Infographic
 njnir.com
njnir.com