Computational chemistry uses computer simulations to model chemical systems and predict molecular behavior, making it essential for designing new materials and drugs. Quantum chemistry specifically applies quantum mechanical principles to understand electron interactions and molecular structures at an atomic level. While computational chemistry encompasses a broader range of modeling techniques, quantum chemistry provides the fundamental theoretical framework for accurate molecular predictions.
Table of Comparison
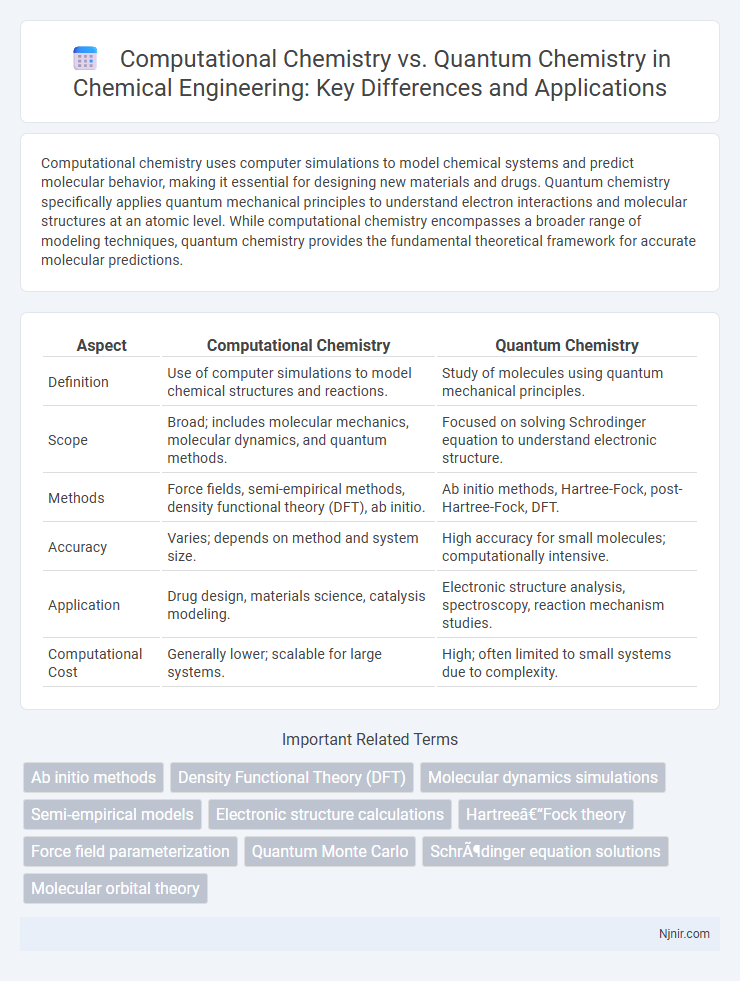
| Aspect | Computational Chemistry | Quantum Chemistry |
|---|---|---|
| Definition | Use of computer simulations to model chemical structures and reactions. | Study of molecules using quantum mechanical principles. |
| Scope | Broad; includes molecular mechanics, molecular dynamics, and quantum methods. | Focused on solving Schrodinger equation to understand electronic structure. |
| Methods | Force fields, semi-empirical methods, density functional theory (DFT), ab initio. | Ab initio methods, Hartree-Fock, post-Hartree-Fock, DFT. |
| Accuracy | Varies; depends on method and system size. | High accuracy for small molecules; computationally intensive. |
| Application | Drug design, materials science, catalysis modeling. | Electronic structure analysis, spectroscopy, reaction mechanism studies. |
| Computational Cost | Generally lower; scalable for large systems. | High; often limited to small systems due to complexity. |
Introduction to Computational and Quantum Chemistry
Computational chemistry uses computer simulations to model and predict chemical structures and reactions, employing methods like molecular mechanics and density functional theory to study molecular systems. Quantum chemistry specifically applies quantum mechanical principles to understand the electronic structure of molecules, solving Schrodinger's equation to calculate properties such as energy levels and molecular orbitals. Both fields intersect in methods that balance computational efficiency with accuracy, enabling detailed analysis of molecular behavior at the atomic level.
Fundamental Principles of Quantum Chemistry
Computational chemistry employs numerical methods and algorithms to model chemical systems, while quantum chemistry is grounded in the fundamental principles of quantum mechanics that describe electron behavior and molecular interactions. Key quantum chemistry concepts include the Schrodinger equation, wave functions, and electron probability distributions, which provide the theoretical framework for accurately predicting molecular properties and reactions. By solving these quantum mechanical equations, quantum chemistry enables precise calculations of energy levels, molecular geometries, and spectroscopic characteristics essential for computational simulations.
Overview of Computational Chemistry Methods
Computational chemistry encompasses a broad range of methods including molecular mechanics, semi-empirical models, density functional theory (DFT), and ab initio techniques, all designed to simulate molecular structures and reactions. Quantum chemistry, a subset of computational chemistry, specifically applies quantum mechanical principles to calculate electronic structures using methods like Hartree-Fock and post-Hartree-Fock approaches. These computational methods vary in accuracy and computational cost, enabling researchers to predict chemical properties and reaction mechanisms with tailored precision.
Key Differences: Computational vs Quantum Chemistry
Computational chemistry encompasses a broad range of numerical methods and simulations used to model chemical systems, whereas quantum chemistry specifically applies quantum mechanics principles to study the electronic structure of molecules. The key difference lies in scope: computational chemistry includes both quantum mechanical and classical approaches like molecular mechanics, while quantum chemistry is strictly focused on solving the Schrodinger equation for atoms and molecules. Quantum chemistry delivers detailed insights into electronic configurations and reaction mechanisms, whereas computational chemistry offers a wider toolkit for predicting molecular properties and dynamics across various scales.
Common Algorithms and Software Tools
Computational chemistry and quantum chemistry both rely on algorithms such as Hartree-Fock, Density Functional Theory (DFT), and post-Hartree-Fock methods like Moller-Plesset perturbation theory (MP2) for molecular simulations and electronic structure calculations. Software tools commonly used in these fields include Gaussian, GAMESS, and ORCA, which provide extensive functionalities for geometry optimization, energy calculation, and spectroscopy prediction. Quantum chemistry emphasizes wavefunction-based techniques, whereas computational chemistry integrates classical and quantum methods to model larger systems and complex chemical processes.
Applications in Chemical Engineering
Computational chemistry employs molecular modeling to optimize catalysts, design new materials, and simulate reaction mechanisms in chemical engineering processes, enhancing efficiency and cost-effectiveness. Quantum chemistry provides fundamental insights into electronic structure and chemical bonding, enabling precise prediction of thermodynamic properties and reaction kinetics critical for designing advanced chemical reactors. Integration of both fields facilitates innovation in process development, enabling accurate simulations of molecular interactions and energy profiles in industrial-scale chemical production.
Accuracy and Limitations of Both Approaches
Computational chemistry encompasses a broad range of methods, including molecular mechanics and semi-empirical techniques, which offer faster but sometimes less accurate results compared to quantum chemistry's ab initio and density functional theory (DFT) approaches known for higher precision in electronic structure calculations. Quantum chemistry provides a detailed quantum mechanical description of molecular systems, enabling accurate predictions of properties such as reaction energies and spectroscopic parameters, but often at the cost of significant computational resources and scalability limitations. Computational chemistry extends applicability to larger systems and longer timescales with approximations that compromise accuracy, while quantum chemistry remains limited to smaller molecules or moderate sizes due to exponential scaling with system complexity.
Role in Molecular Modeling and Simulation
Computational chemistry utilizes classical mechanics and empirical models to simulate molecular behavior, enabling efficient analysis of large biomolecules and complex systems. Quantum chemistry provides a rigorous, first-principles approach based on quantum mechanics to accurately predict electronic structure and chemical properties at the atomic level. Together, these methods complement each other in molecular modeling, with computational chemistry offering scalability and quantum chemistry ensuring precise electronic insights for reaction mechanisms and spectroscopy.
Future Trends in Computational and Quantum Chemistry
Future trends in computational and quantum chemistry emphasize the integration of machine learning algorithms to enhance predictive accuracy and reduce computational costs. Advances in quantum computing hardware are expected to revolutionize quantum chemistry by enabling simulations of complex molecular systems beyond classical limits. The development of hybrid quantum-classical methods will accelerate drug discovery and materials science through more precise modeling of electron interactions and chemical reactions.
Choosing the Right Approach for Engineering Problems
Computational chemistry uses classical algorithms and molecular mechanics to model large-scale chemical systems efficiently, while quantum chemistry employs quantum mechanics to accurately describe electronic structures at the atomic level. Engineering problems requiring precise predictions of molecular interactions, reaction pathways, or electronic properties benefit from quantum chemistry methods such as density functional theory (DFT) or ab initio calculations. For complex materials design and large biomolecular simulations, computational chemistry techniques like molecular dynamics and Monte Carlo simulations provide scalable and practical solutions.
Ab initio methods
Ab initio methods in computational chemistry rely on quantum chemistry principles to accurately predict molecular properties by solving the Schrodinger equation without empirical parameters.
Density Functional Theory (DFT)
Density Functional Theory (DFT) is a key quantum chemistry method within computational chemistry, enabling accurate electronic structure calculations by modeling electron density rather than wavefunctions.
Molecular dynamics simulations
Computational chemistry employs molecular dynamics simulations to study molecular behavior over time, while quantum chemistry provides electronic structure calculations essential for accurate force fields in these simulations.
Semi-empirical models
Semi-empirical models in computational chemistry utilize quantum mechanical principles combined with empirical parameters to efficiently approximate molecular electronic structures and properties.
Electronic structure calculations
Electronic structure calculations in computational chemistry use quantum chemistry methods to model molecular orbitals and electron interactions with high precision, enabling accurate prediction of chemical properties and reactions.
Hartree–Fock theory
Hartree-Fock theory serves as a fundamental computational chemistry method for approximating the quantum states of electrons in atoms and molecules, bridging practical calculations and quantum chemistry principles.
Force field parameterization
Force field parameterization in computational chemistry involves approximating molecular interactions using classical potentials, whereas quantum chemistry derives parameters from first-principles electronic structure calculations to improve accuracy.
Quantum Monte Carlo
Quantum Monte Carlo methods in quantum chemistry provide highly accurate solutions to the Schrodinger equation by stochastically sampling electron configurations, surpassing many traditional computational chemistry techniques in precision and scalability for complex molecular systems.
Schrödinger equation solutions
Computational chemistry uses numerical methods to approximate Schrodinger equation solutions for molecular systems, while quantum chemistry focuses on rigorous theoretical formulations and exact or near-exact solutions of the Schrodinger equation to understand atomic and molecular behavior.
Molecular orbital theory
Molecular orbital theory, a fundamental concept in quantum chemistry, provides a more detailed and accurate description of electron behavior in molecules compared to the approximations commonly used in computational chemistry.
Computational chemistry vs quantum chemistry Infographic
 njnir.com
njnir.com