Electrochemical synthesis offers precise control over reaction conditions and operates under milder temperatures and pressures compared to thermochemical synthesis, enhancing energy efficiency and selectivity. Thermochemical synthesis often relies on high temperatures and catalysts to drive reactions, which can lead to broader product distribution and higher energy consumption. Integrating electrochemical methods can reduce environmental impact through lower greenhouse gas emissions and enable sustainable production of chemicals.
Table of Comparison
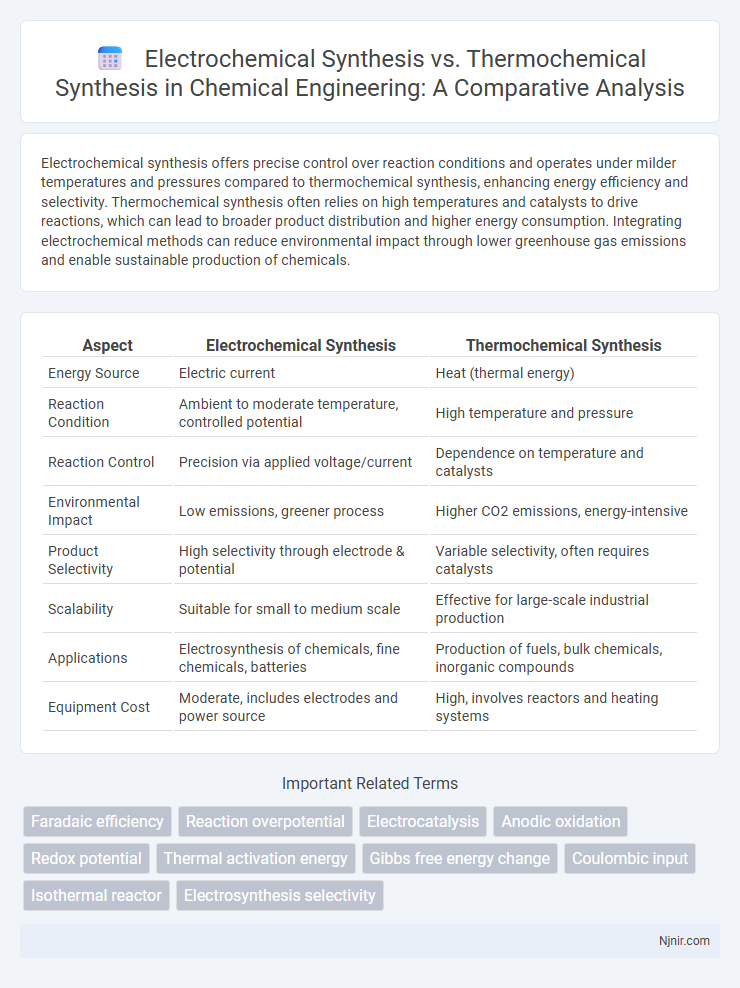
| Aspect | Electrochemical Synthesis | Thermochemical Synthesis |
|---|---|---|
| Energy Source | Electric current | Heat (thermal energy) |
| Reaction Condition | Ambient to moderate temperature, controlled potential | High temperature and pressure |
| Reaction Control | Precision via applied voltage/current | Dependence on temperature and catalysts |
| Environmental Impact | Low emissions, greener process | Higher CO2 emissions, energy-intensive |
| Product Selectivity | High selectivity through electrode & potential | Variable selectivity, often requires catalysts |
| Scalability | Suitable for small to medium scale | Effective for large-scale industrial production |
| Applications | Electrosynthesis of chemicals, fine chemicals, batteries | Production of fuels, bulk chemicals, inorganic compounds |
| Equipment Cost | Moderate, includes electrodes and power source | High, involves reactors and heating systems |
Introduction to Chemical Synthesis Methods
Electrochemical synthesis utilizes electrical energy to drive chemical reactions, enabling precise control over reaction conditions and product selectivity, making it ideal for sustainable and energy-efficient chemical production. Thermochemical synthesis relies on heat to initiate and sustain chemical transformations, often involving higher temperatures and pressures, which are suitable for bulk manufacturing and industrial-scale processes. Both methods offer distinct advantages depending on the desired reaction pathway, energy consumption, and environmental impact in chemical synthesis applications.
Fundamentals of Electrochemical Synthesis
Electrochemical synthesis involves driving chemical reactions through the application of electrical energy, utilizing electrodes to facilitate redox processes under controlled potential and current. Unlike thermochemical synthesis, which relies on heat to overcome activation energy barriers, electrochemical methods offer precise control over reaction pathways, enabling selective and efficient transformations at ambient conditions. Key fundamentals include the design of electrodes, electrolyte composition, and the interplay of thermodynamics and kinetics that govern electron transfer reactions at the electrode-electrolyte interface.
Principles of Thermochemical Synthesis
Thermochemical synthesis relies on heat-driven chemical reactions, often involving high temperatures and pressures to convert reactants into desired products through bond breaking and formation. This method utilizes classical thermodynamics principles, such as enthalpy and entropy changes, to predict reaction feasibility and optimize conditions for maximum yield. Common applications include catalytic reforming and combustion processes where thermal energy directly drives chemical transformations.
Reaction Mechanisms: Electrochemical vs Thermochemical
Electrochemical synthesis involves electron transfer through electrodes, enabling precise control over redox reactions and reaction intermediates at the electrode surface, which differs from thermochemical synthesis that relies on heat-induced bond breaking and formation driven by thermal energy. In electrochemical reactions, the potential applied directly influences reaction pathways and selectivity, while thermochemical mechanisms depend on temperature and pressure to overcome activation barriers and dictate product distribution. The distinct energy sources in these methods result in fundamentally different reaction mechanisms, enabling electrochemical synthesis to access reaction routes unattainable by traditional thermochemical approaches.
Energy Efficiency Comparison
Electrochemical synthesis generally offers higher energy efficiency compared to thermochemical synthesis by enabling precise control over reaction conditions and minimizing energy loss through heat dissipation. Thermochemical methods typically require elevated temperatures and pressures, resulting in substantial energy consumption and lower overall efficiency. Advances in electrochemical technologies, such as renewable electricity sources and enhanced electrode materials, further improve their energy efficiency advantages in chemical manufacturing.
Environmental Impact Assessment
Electrochemical synthesis offers significant environmental advantages over thermochemical synthesis by operating at lower temperatures and pressures, reducing energy consumption and greenhouse gas emissions. The use of renewable electricity in electrochemical processes further decreases carbon footprint, while thermochemical methods typically rely on fossil fuels, contributing to higher CO2 emissions and air pollutants. Life cycle assessments indicate that electrochemical synthesis generates less hazardous waste and offers better potential for carbon-neutral chemical production.
Feedstock and Raw Material Flexibility
Electrochemical synthesis offers significant advantages in feedstock and raw material flexibility by directly utilizing electricity to drive reactions, enabling the use of a wide range of renewable or alternative inputs such as CO2, water, and nitrogen under mild conditions. Thermochemical synthesis traditionally relies on fossil-based feedstocks and high temperatures, often limiting raw material diversity and increasing dependence on non-renewable resources. The ability of electrochemical methods to convert diverse feedstocks into valuable chemicals with lower energy consumption enhances sustainability and adaptability in chemical manufacturing.
Process Scalability and Industrial Applications
Electrochemical synthesis offers high precision and energy efficiency at moderate temperatures, making it ideal for scalable production of specialty chemicals and fine materials in industries such as pharmaceuticals and electronics. Thermochemical synthesis operates at high temperatures with widely established processes, enabling large-scale manufacturing of bulk chemicals like ammonia and methanol but often requires significant energy input and complex reactors. Industrial applications favor electrochemical methods for sustainability and selectivity, while thermochemical routes dominate where robust, high-throughput output is critical.
Technological Challenges and Limitations
Electrochemical synthesis faces technological challenges such as electrode material degradation, limited current efficiency, and scaling difficulties for industrial applications. Thermochemical synthesis is constrained by high energy consumption, temperature control complexities, and often requires expensive catalysts to achieve desired selectivity. Both methods face limitations in reaction specificity and product purity, impacting overall process efficiency and commercial viability.
Future Trends in Chemical Synthesis Technologies
Electrochemical synthesis offers precise control over reaction conditions, enabling energy-efficient and environmentally friendly pathways compared to traditional thermochemical methods that rely on high temperatures and pressures. Future trends emphasize the integration of renewable electricity sources with electrochemical reactors to reduce carbon footprints and enhance sustainability in chemical manufacturing. Advances in catalyst development and reactor design are expected to further boost the scalability and selectivity of electrochemical synthesis, positioning it as a key technology in the shift toward green chemistry.
Faradaic efficiency
Electrochemical synthesis achieves higher Faradaic efficiency than thermochemical synthesis by directly utilizing electron transfer for targeted chemical transformations with minimal energy loss.
Reaction overpotential
Electrochemical synthesis exhibits lower reaction overpotential compared to thermochemical synthesis, enhancing energy efficiency and selectivity in chemical reactions.
Electrocatalysis
Electrocatalysis in electrochemical synthesis offers higher selectivity and energy efficiency compared to traditional thermochemical synthesis by enabling precise control of reaction pathways at the electrode interface.
Anodic oxidation
Anodic oxidation in electrochemical synthesis offers precise control over reaction pathways and product selectivity compared to thermochemical synthesis, enabling efficient and environmentally friendly production of oxidized compounds.
Redox potential
Electrochemical synthesis offers precise control over redox potential through applied voltage, enabling selective redox reactions, while thermochemical synthesis relies on temperature-driven redox equilibria with less tunable redox potential.
Thermal activation energy
Electrochemical synthesis requires significantly lower thermal activation energy compared to thermochemical synthesis, enabling more energy-efficient chemical transformations at milder temperatures.
Gibbs free energy change
Electrochemical synthesis often enables reactions with positive Gibbs free energy change by applying electrical energy, whereas thermochemical synthesis relies on temperature-driven reactions where Gibbs free energy change must be negative for spontaneity.
Coulombic input
Electrochemical synthesis requires significantly lower Coulombic input compared to thermochemical synthesis, enhancing energy efficiency by directly utilizing electric current to drive chemical reactions.
Isothermal reactor
Isothermal reactors in electrochemical synthesis enable precise temperature control for enhanced reaction selectivity and energy efficiency compared to the higher energy demands and temperature gradients often observed in thermochemical synthesis.
Electrosynthesis selectivity
Electrochemical synthesis offers superior electrosynthesis selectivity compared to thermochemical methods by precisely controlling reaction pathways through applied potentials and electrode materials.
Electrochemical synthesis vs thermochemical synthesis Infographic
 njnir.com
njnir.com